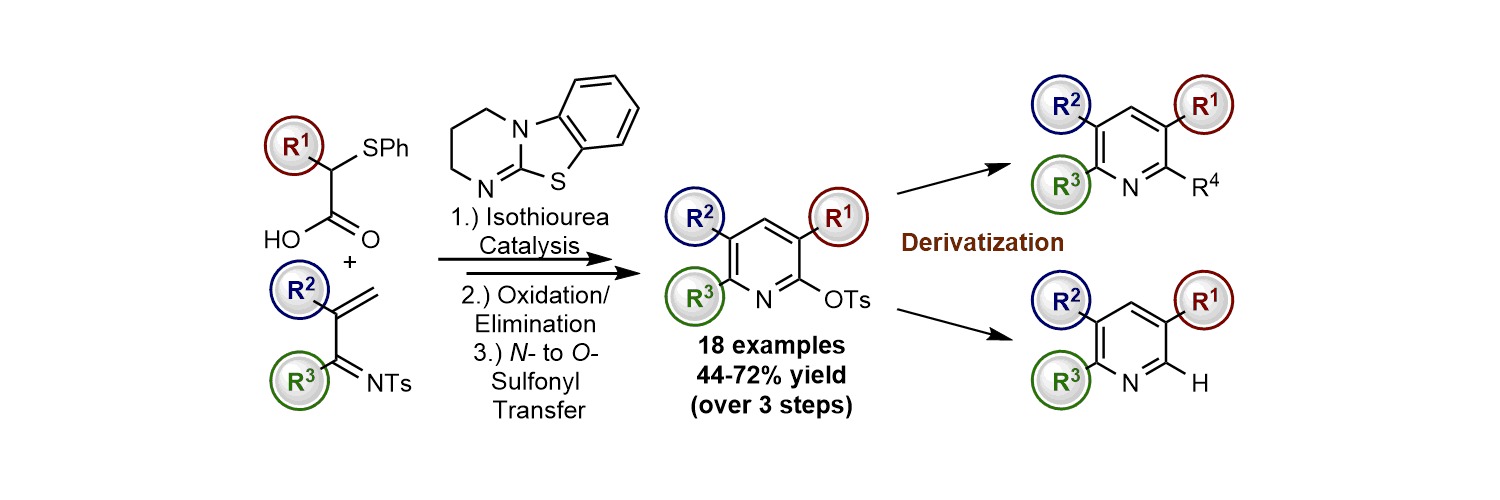
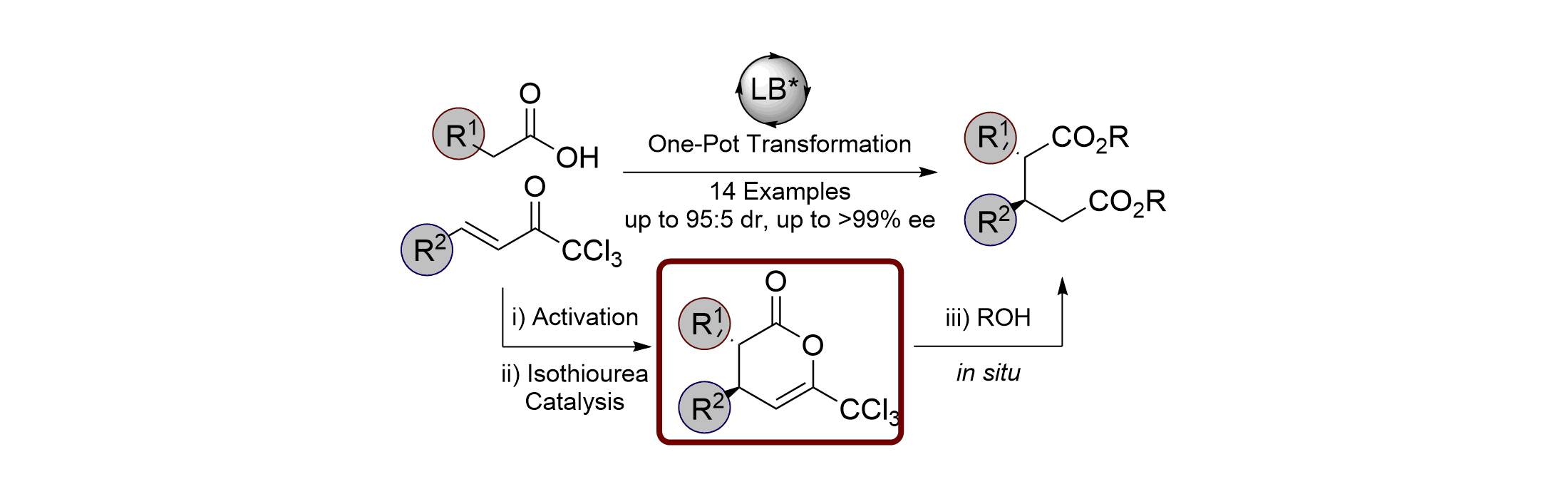
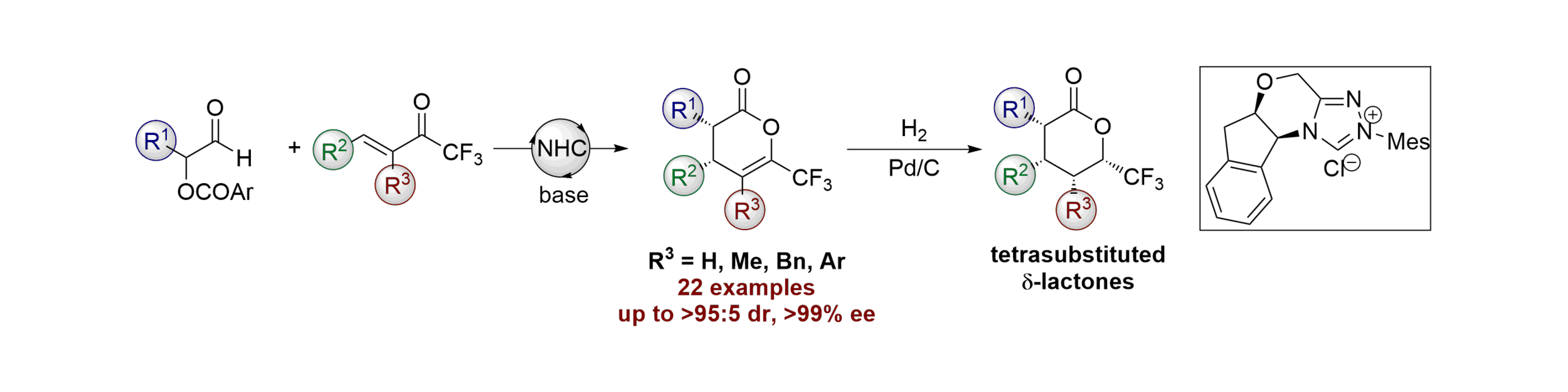
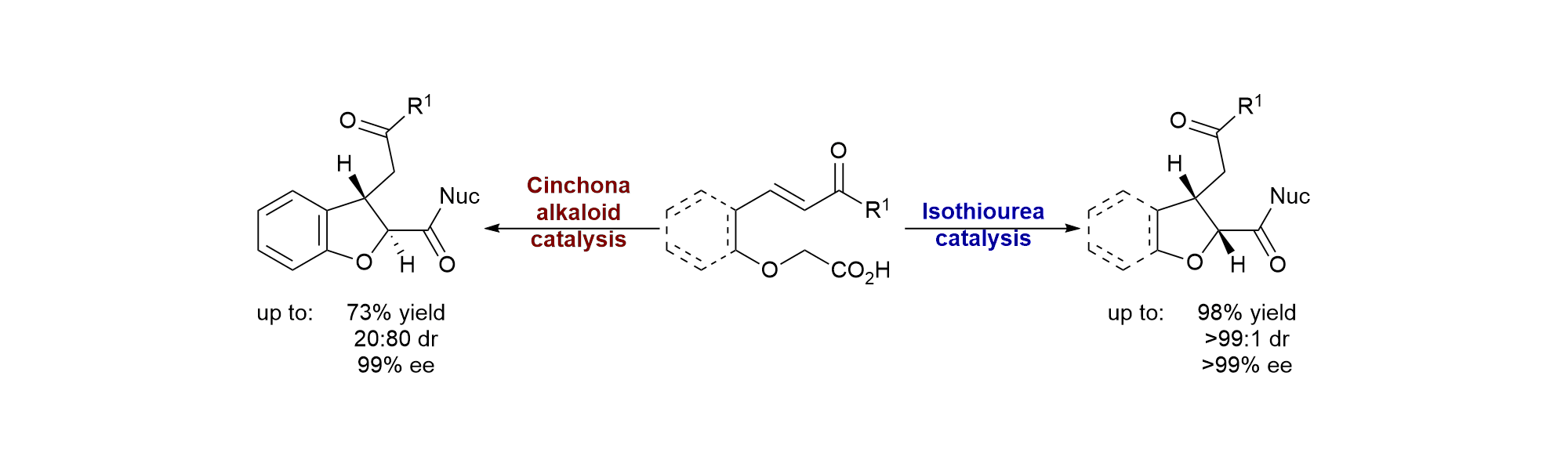
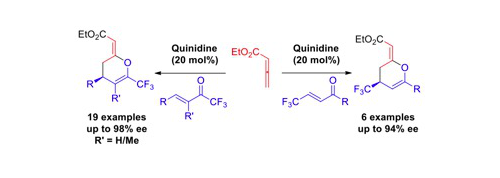Publications
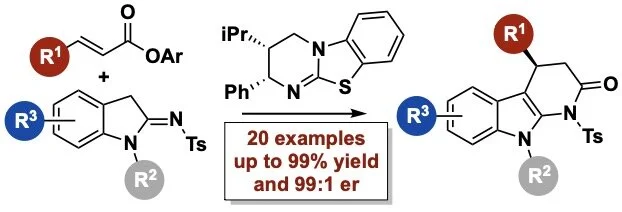
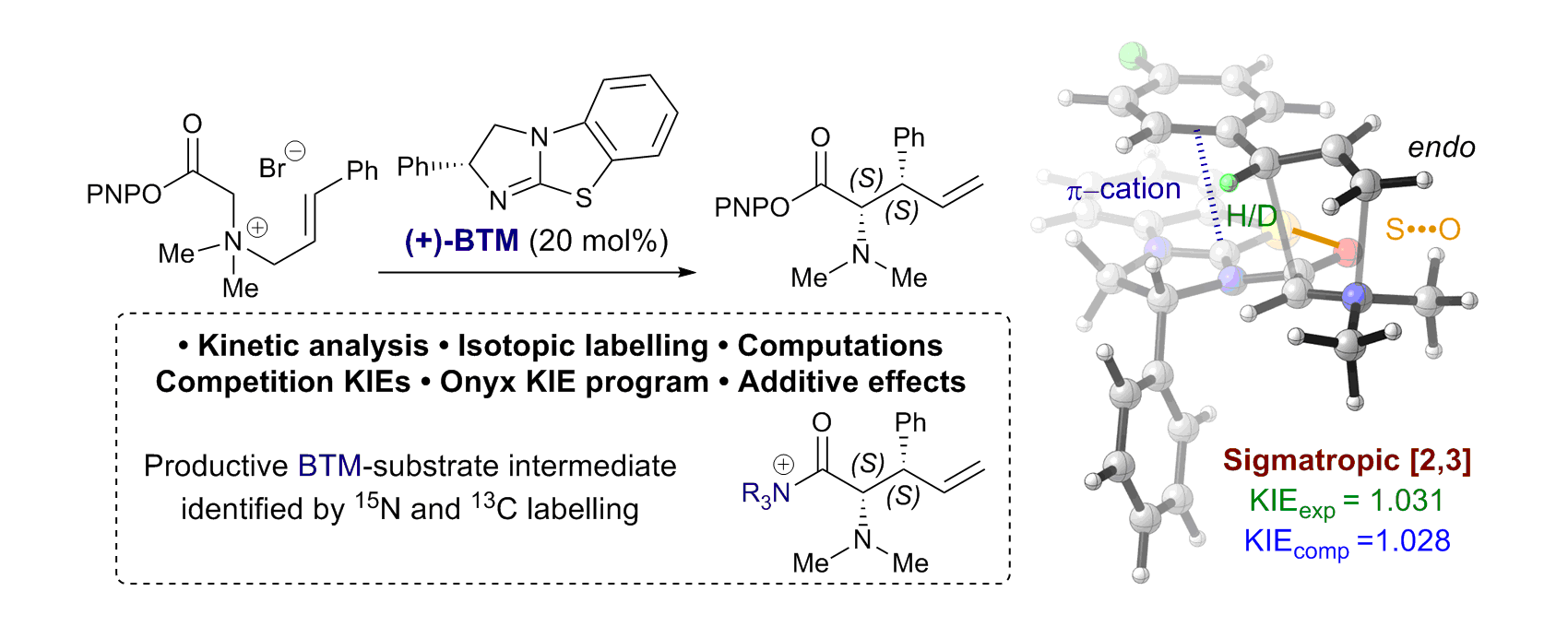
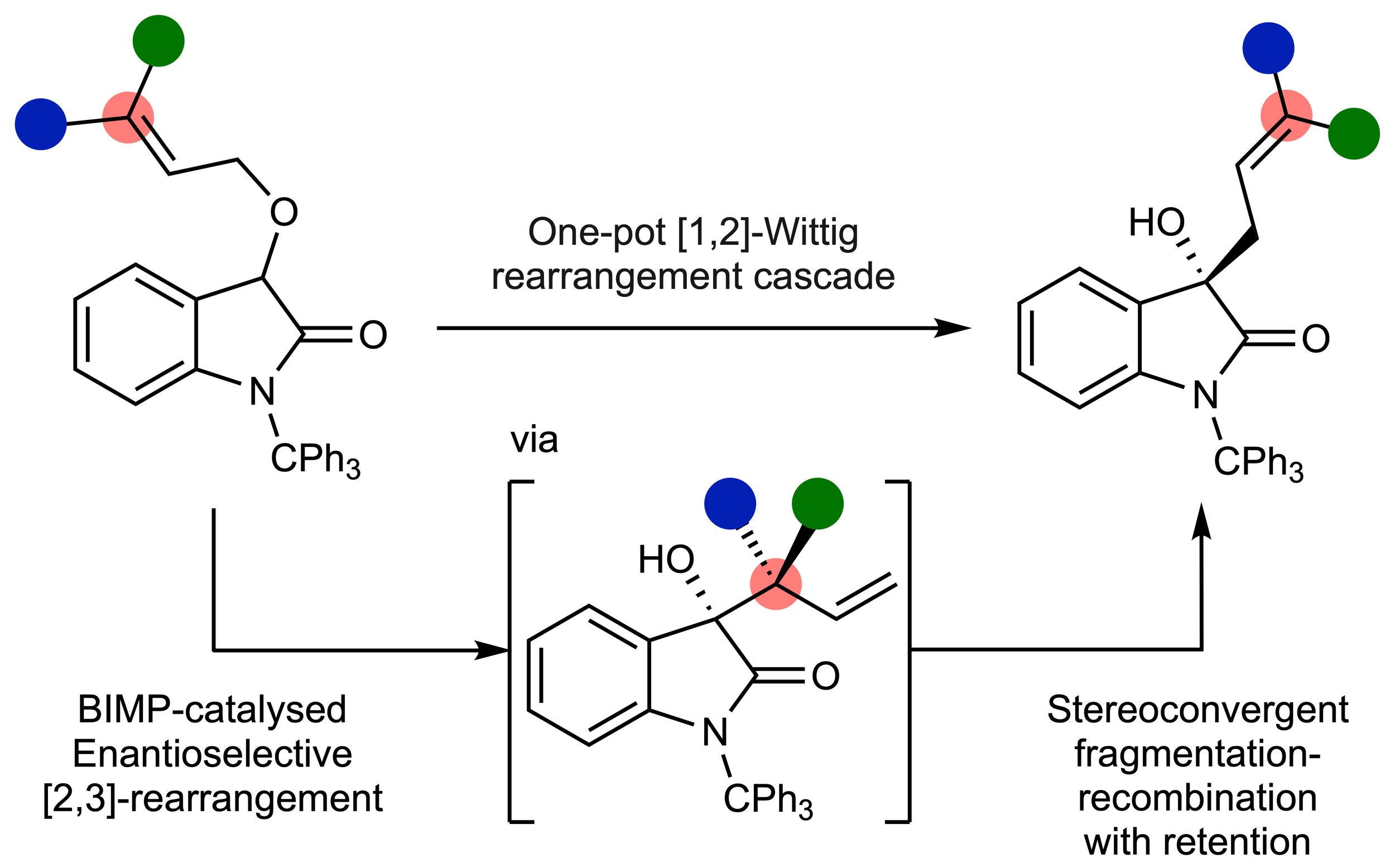
170. The catalytic enantioselective [1,2]-Wittig rearrangement cascade of allylic ethers
T. Kang, J. O’Yang, K. Kasten. S. S. Allsop, T. Lewis-Atwell, E. H. Farrar, M. Juhl,. D. B. Cordes, A. P. McKay, M. N. Grayson,* A. D. Smith*
Nature Chemistry, 2026, https://doi.org/10.1038/s41557-025-02022-4
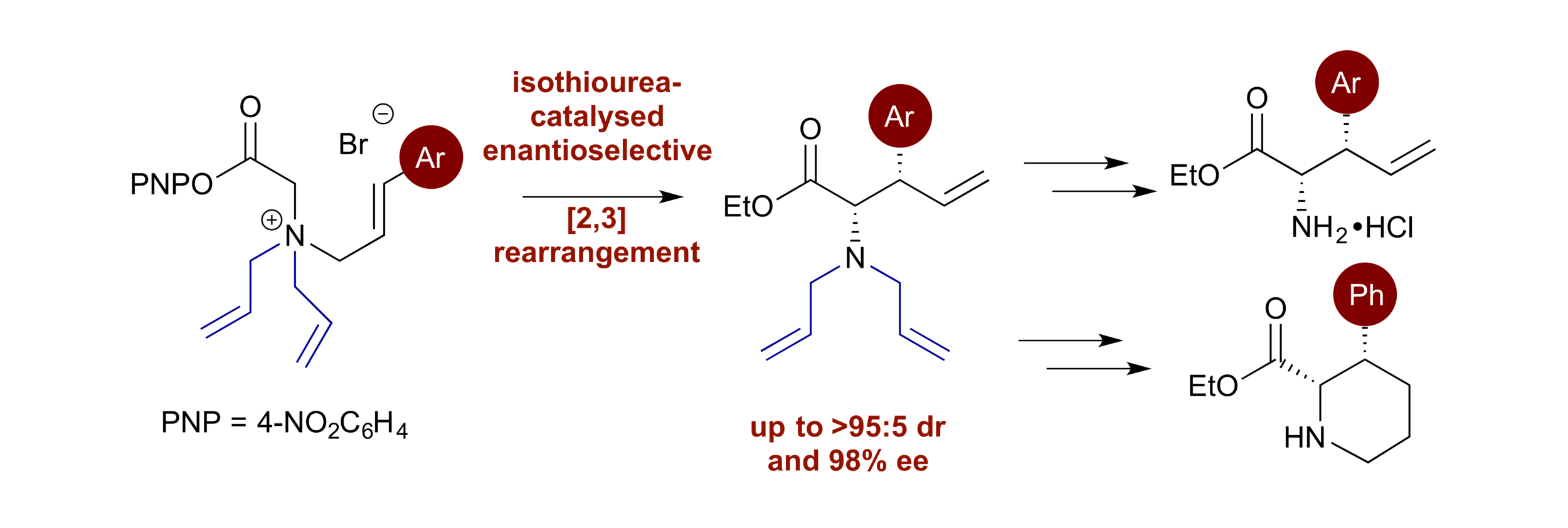
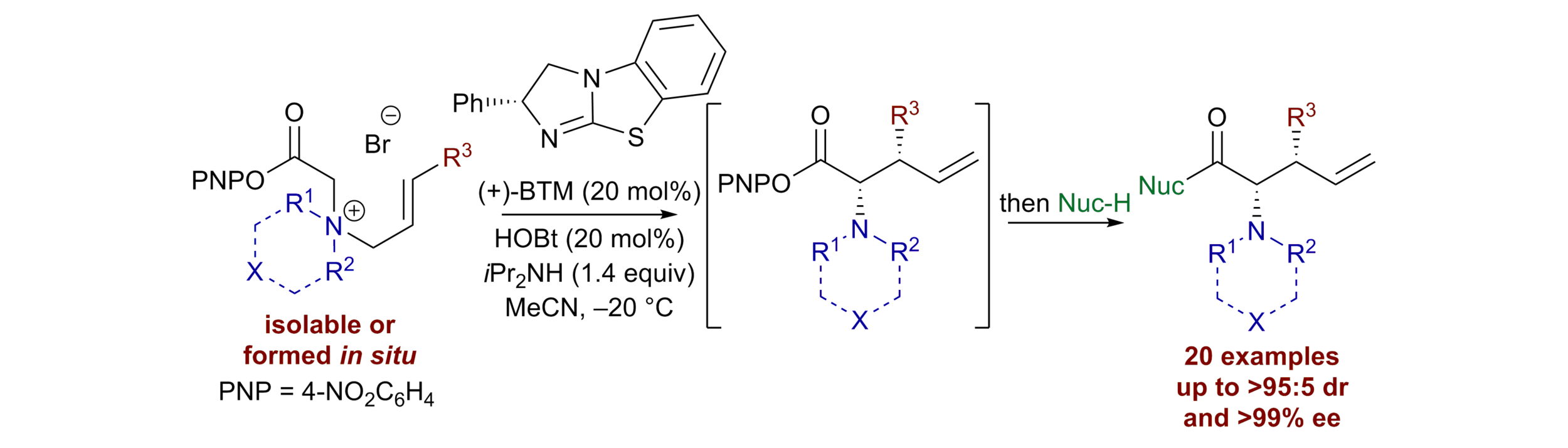
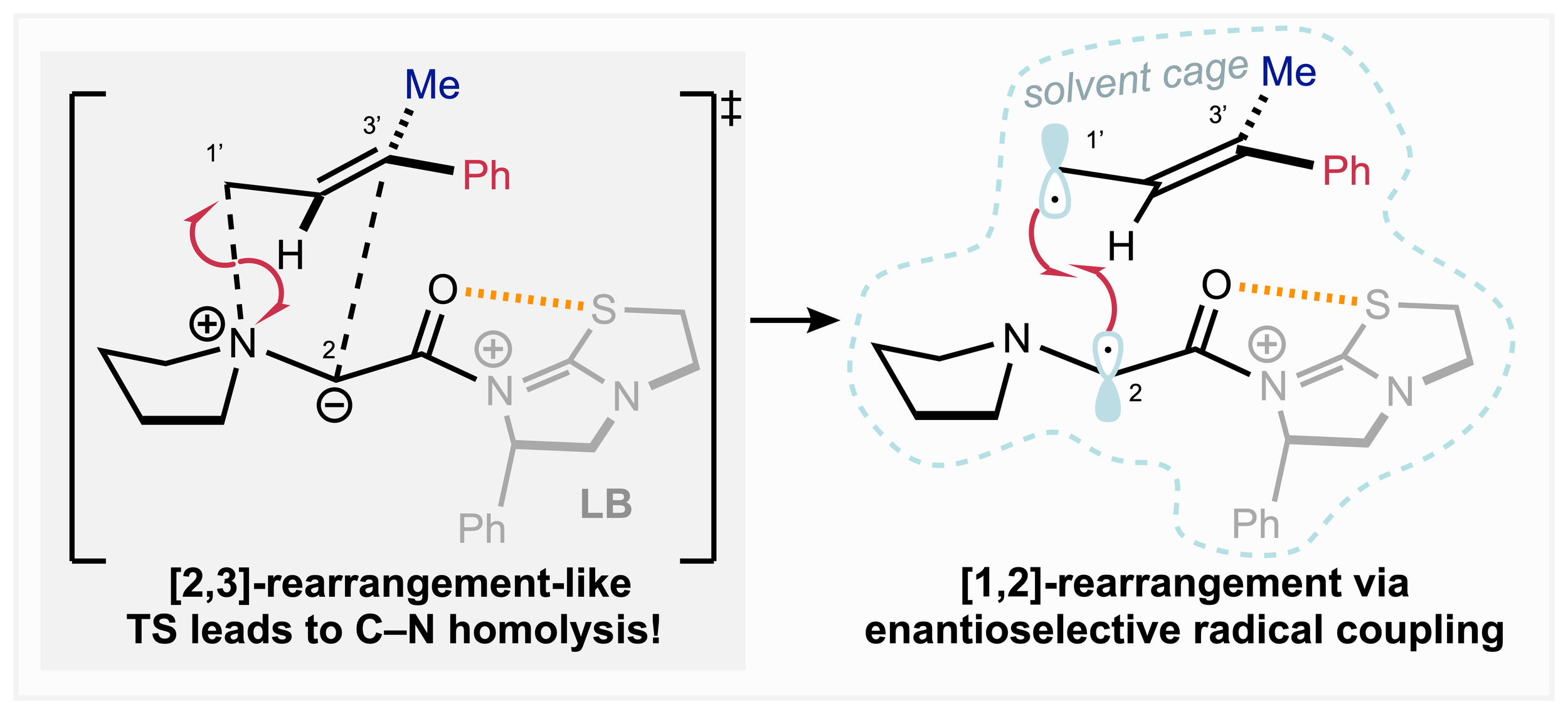
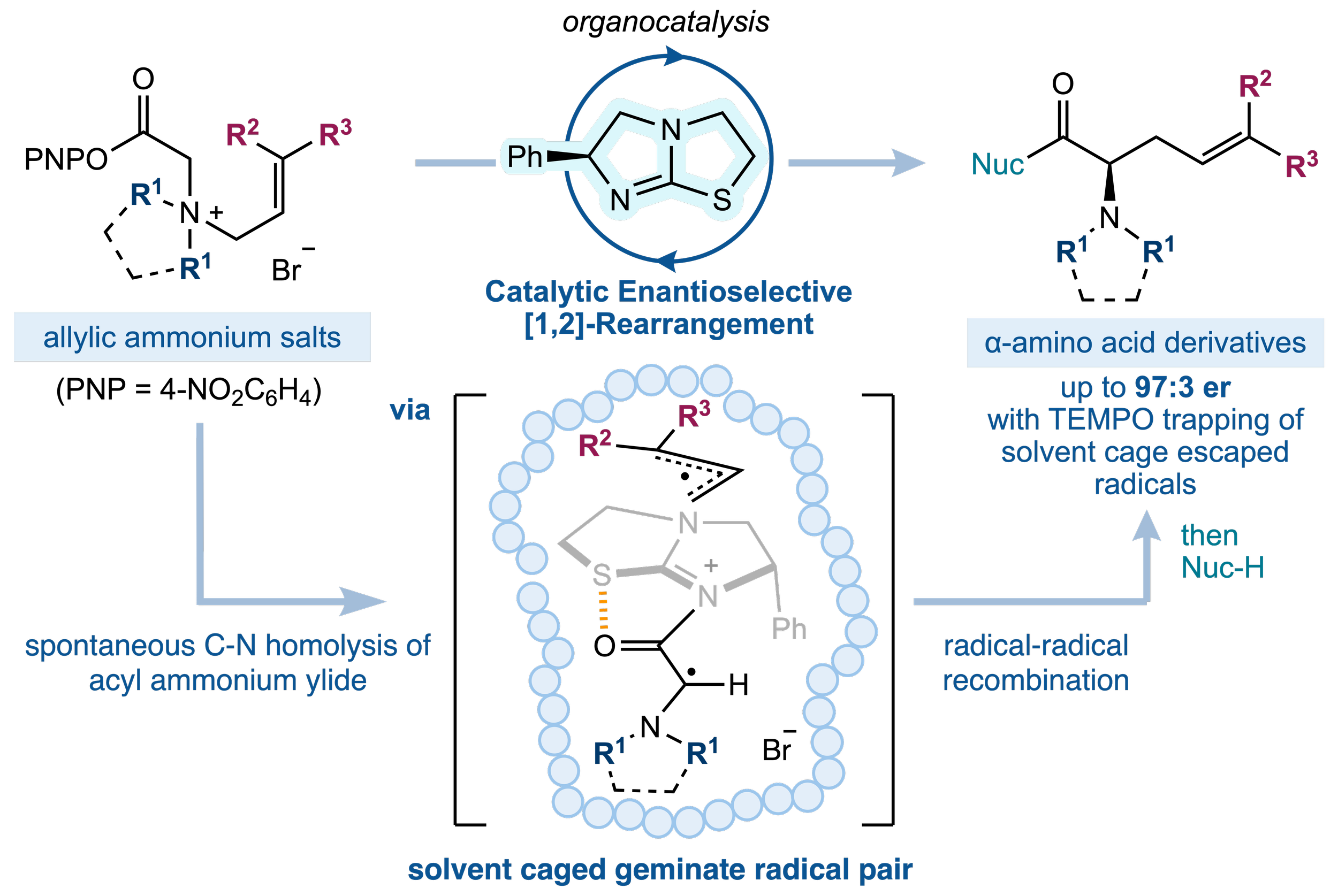
169. Enantiocontrol in Radical Coupling Reactions: A Catalytic [1,2]-Rearrangement of Allylic Ammonium Ylides
W. Hartley,* K. Kasten* and A. D. Smith
Synlett, 2025, accepted for publication, https://doi.org/10.1055/a-2702-3605
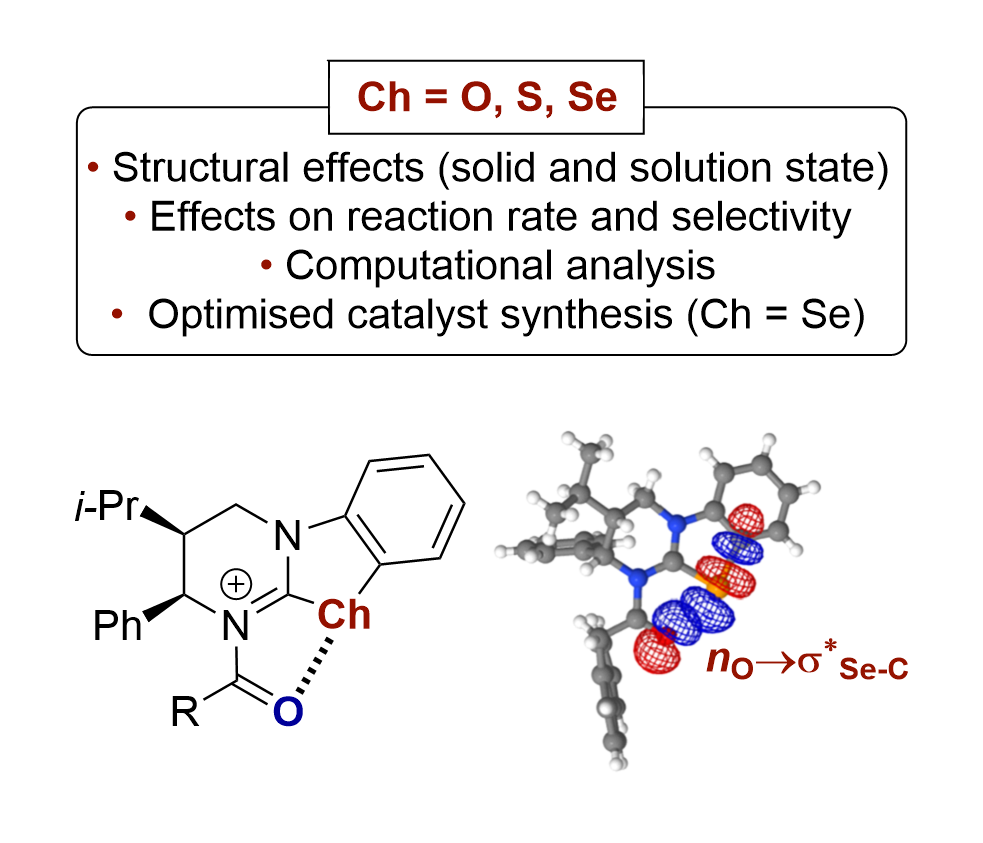
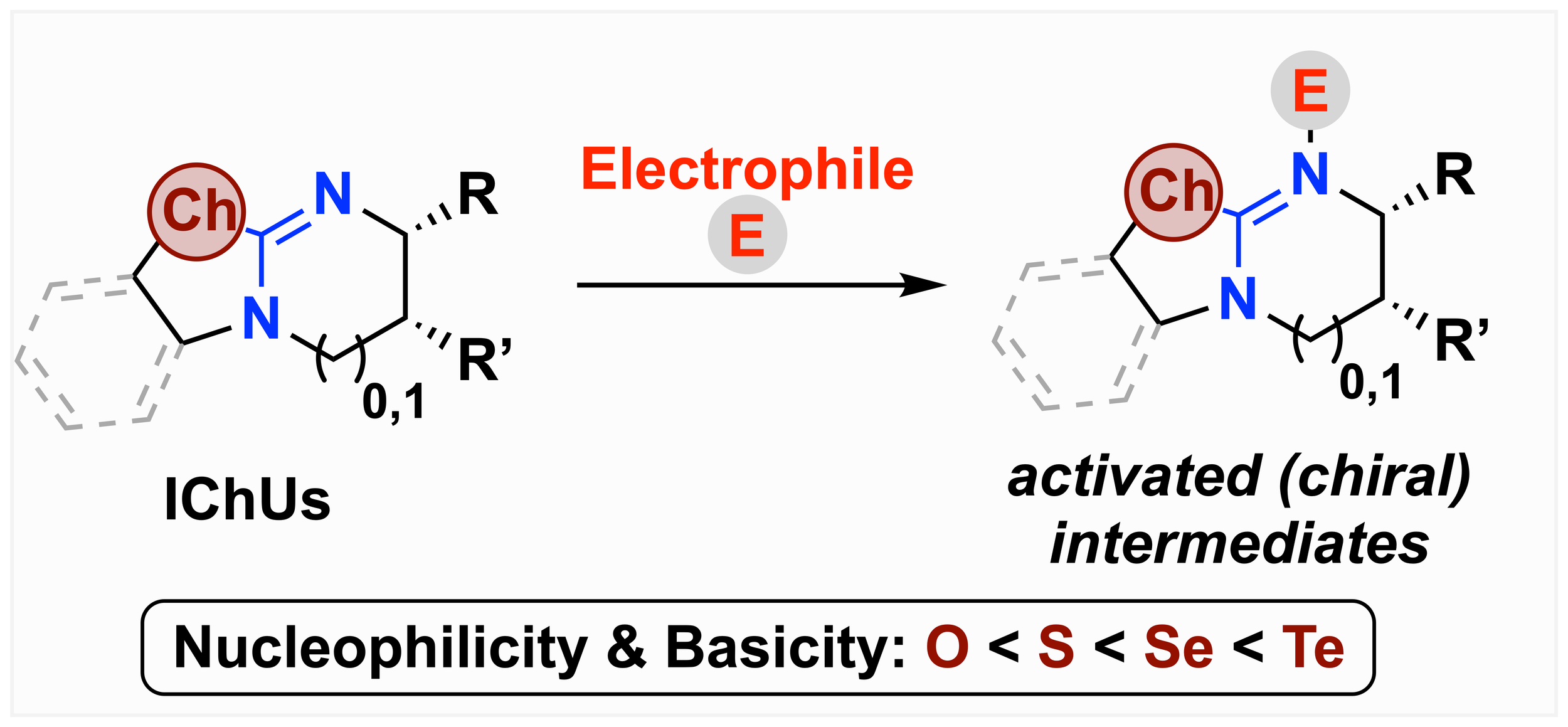
168. From Oxygen to Tellurium: The Impact of the Chalcogen on Nucleophilicities and Basicities of Isochalcogenourea Catalysts
L. Stockhammer, K. Kasten, A. Eitzinger, L. S. Vogl, M. Piringer, D. Weinzierl, A. R. Ofial, * A. D. Smith,* and M. Waser*
Angew. Chem. Int. Ed. 2025, e202514865, accepted for publication.
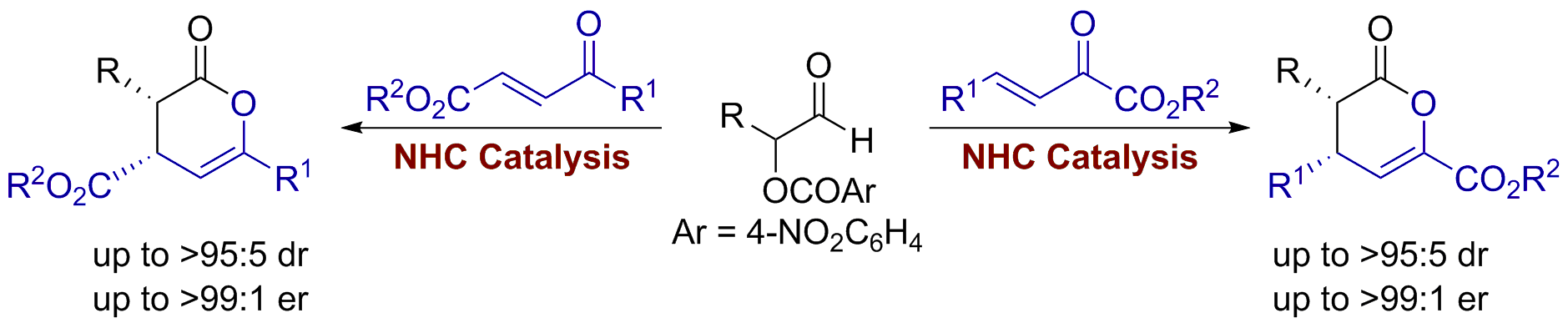
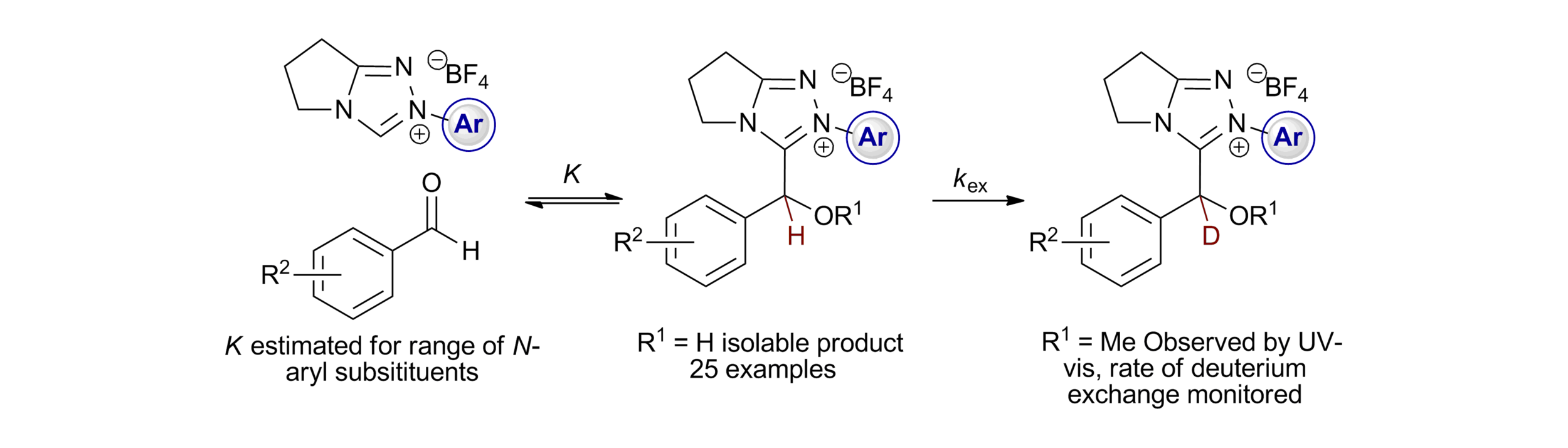
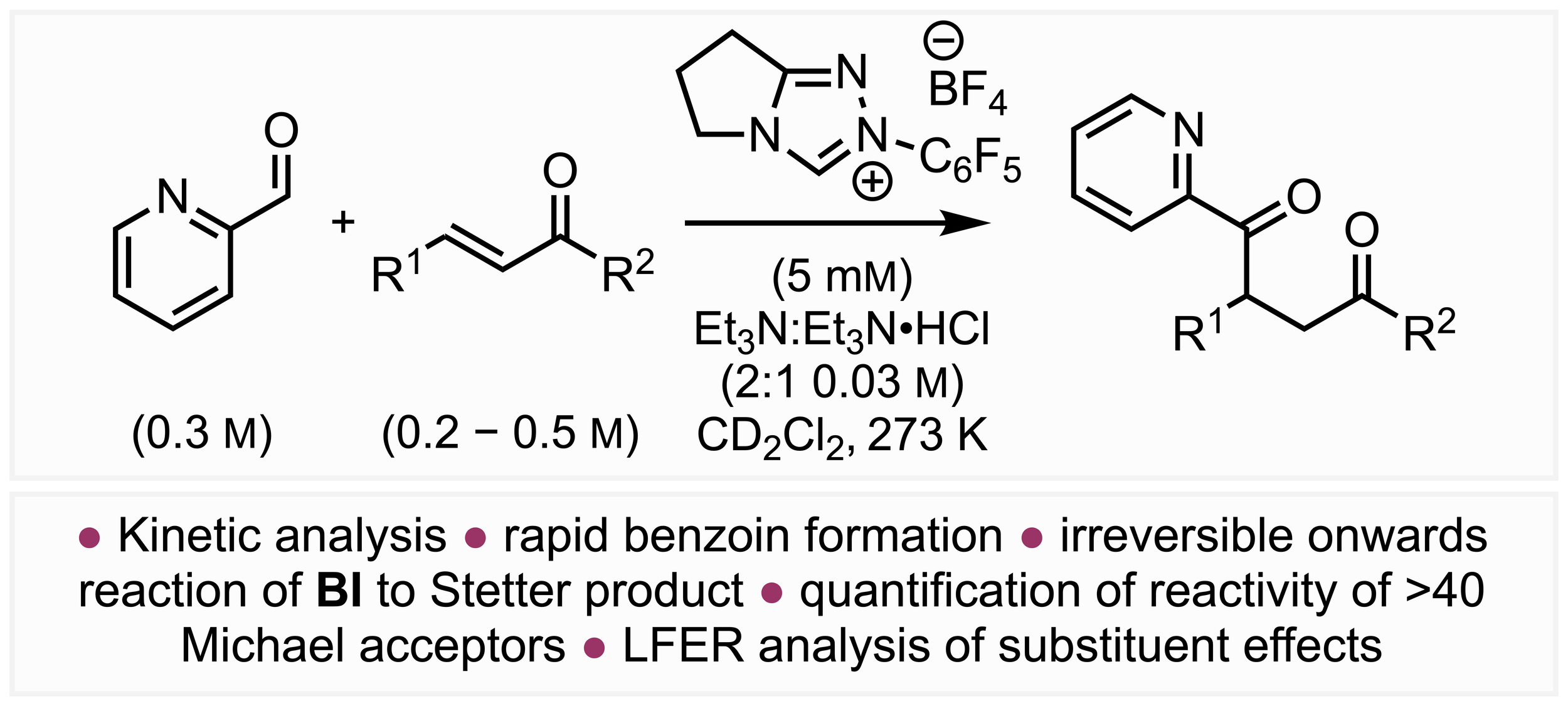
167. Quantifying Breslow Intermediate Reactivity in Intermolecular Stetter Reactions
Z. Duan, J. Zhu, P. K. Majhi, A. S. Goodfellow,* A. C. O’Donoghue,* C. M. Young* and A D. Smith,*
Chem. Sci. 2025, 16, accepted for publication, https://doi.org/10.1039/D5SC05021A
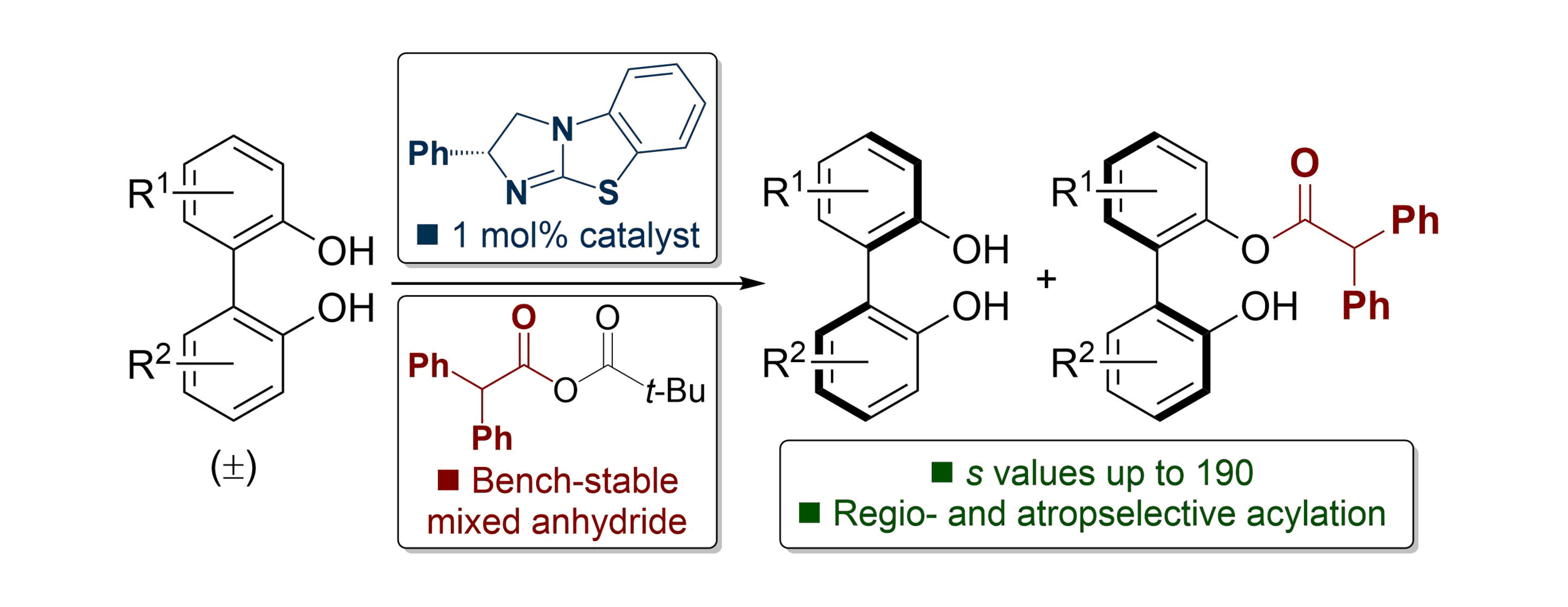
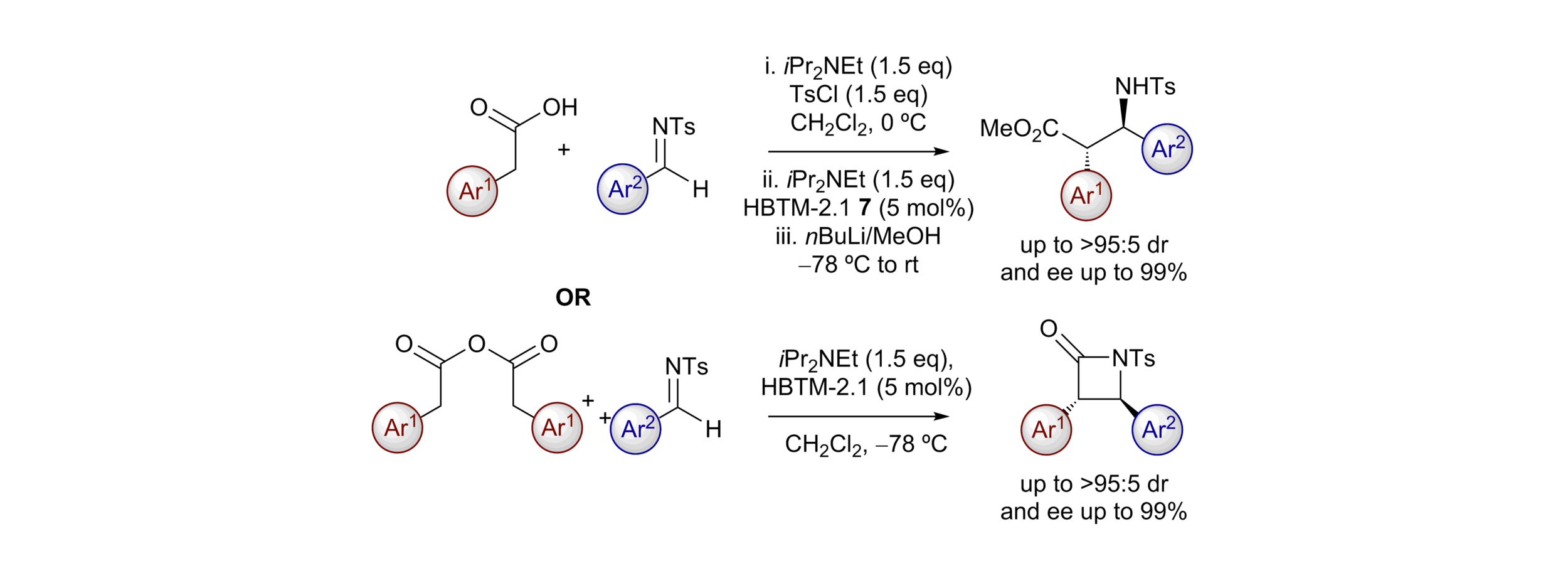
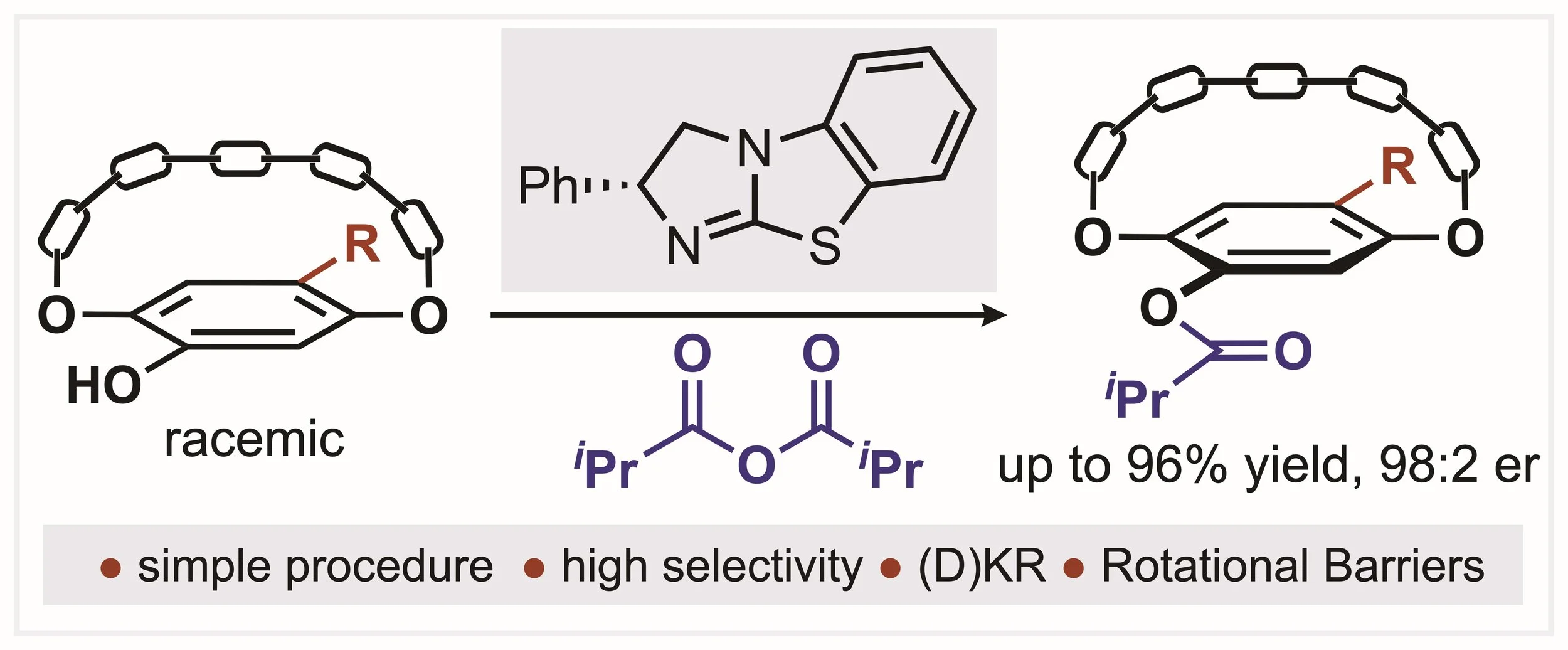
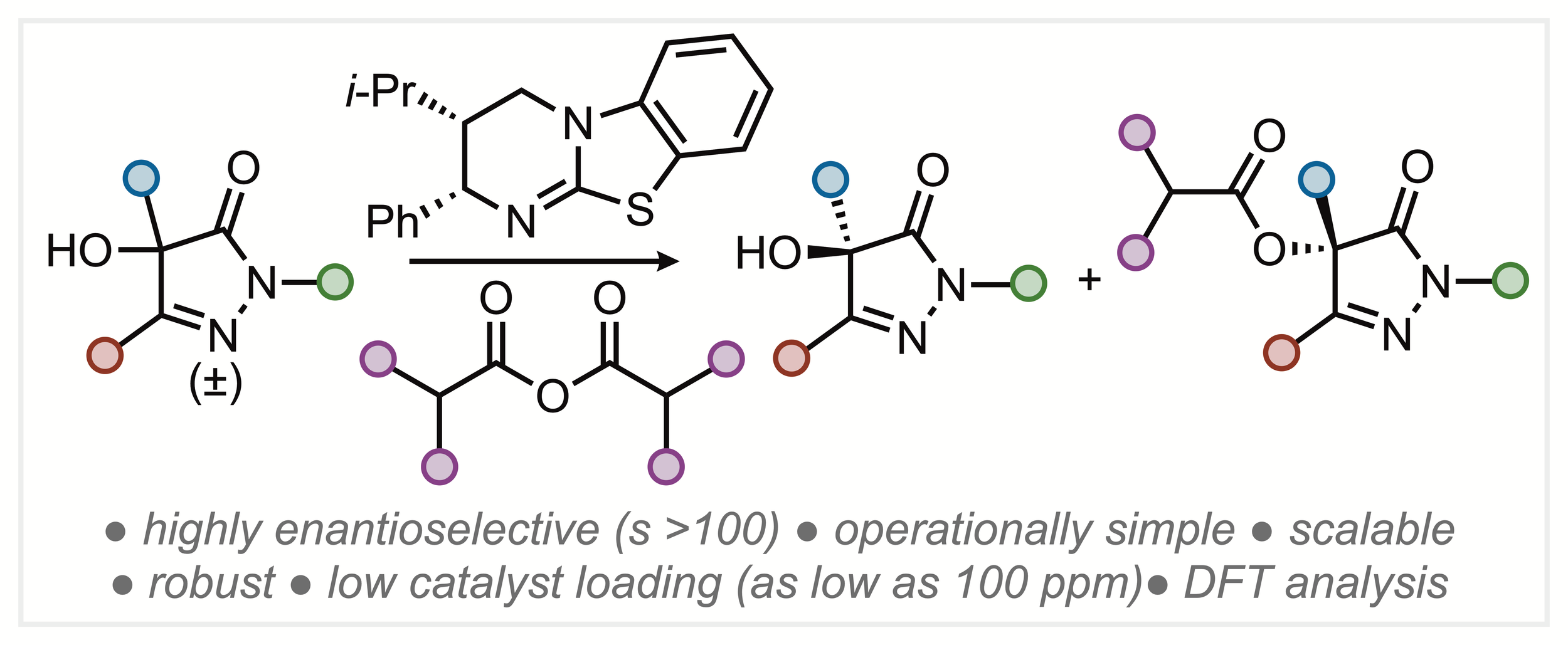
166. Isothiourea-Catalysed Acylative Kinetic and Dynamic Kinetic Resolution of Planar Chiral Paracyclophanols
Z. Zhou, K. Kasten, A. P. McKay, D. B. Cordes and A. D. Smith
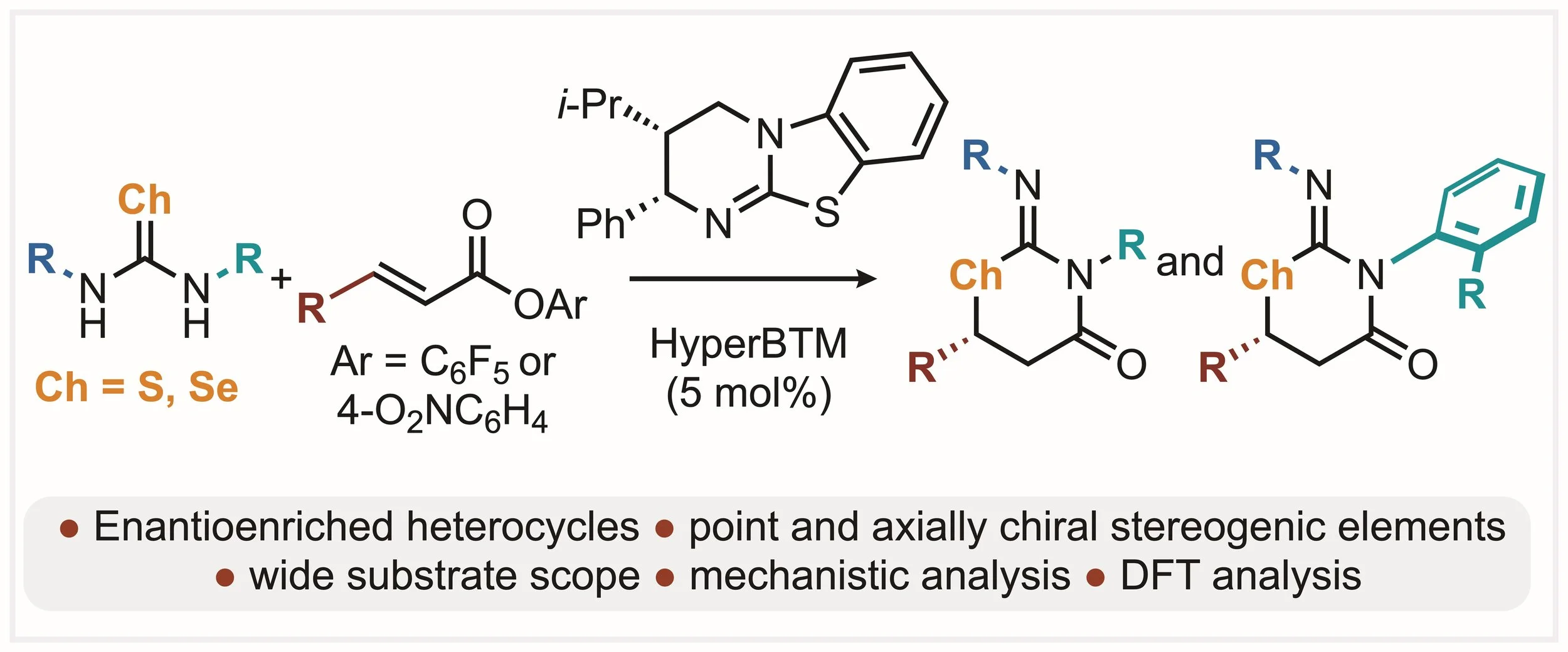
165. Isothiourea Catalysed Enantioselective Generation of Point and Axially Chiral Iminothia- and Iminoselenazinanones
A. J. Nimmo, A. S. Goodfellow, J. T. Guntley, A. P. McKay, D. B. Cordes, M. Bühl and A. D. Smith
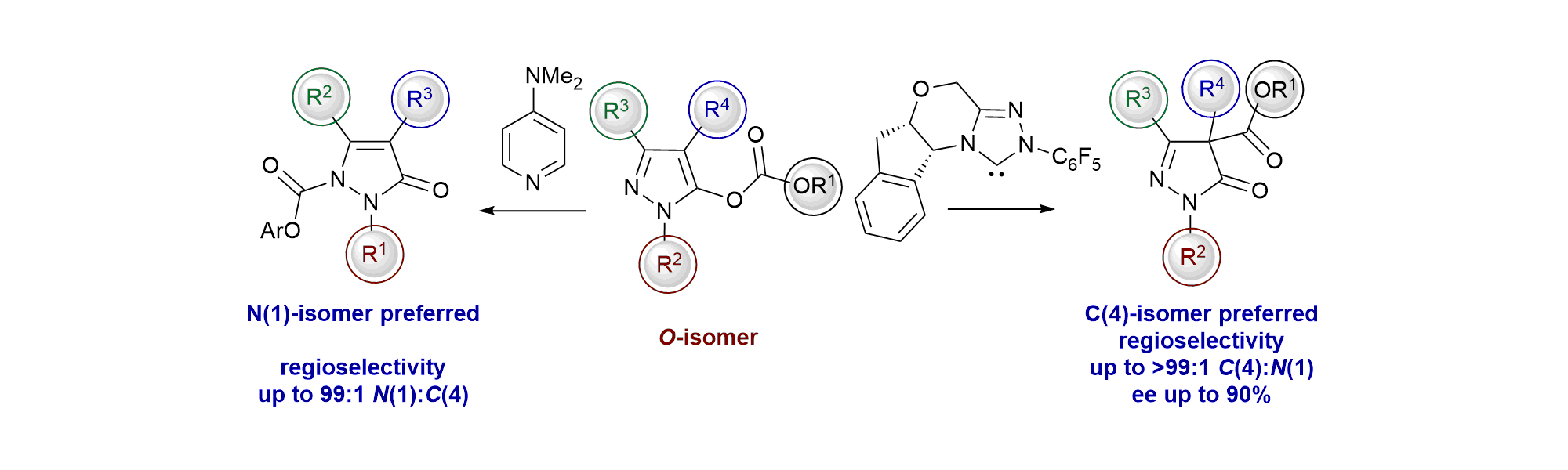
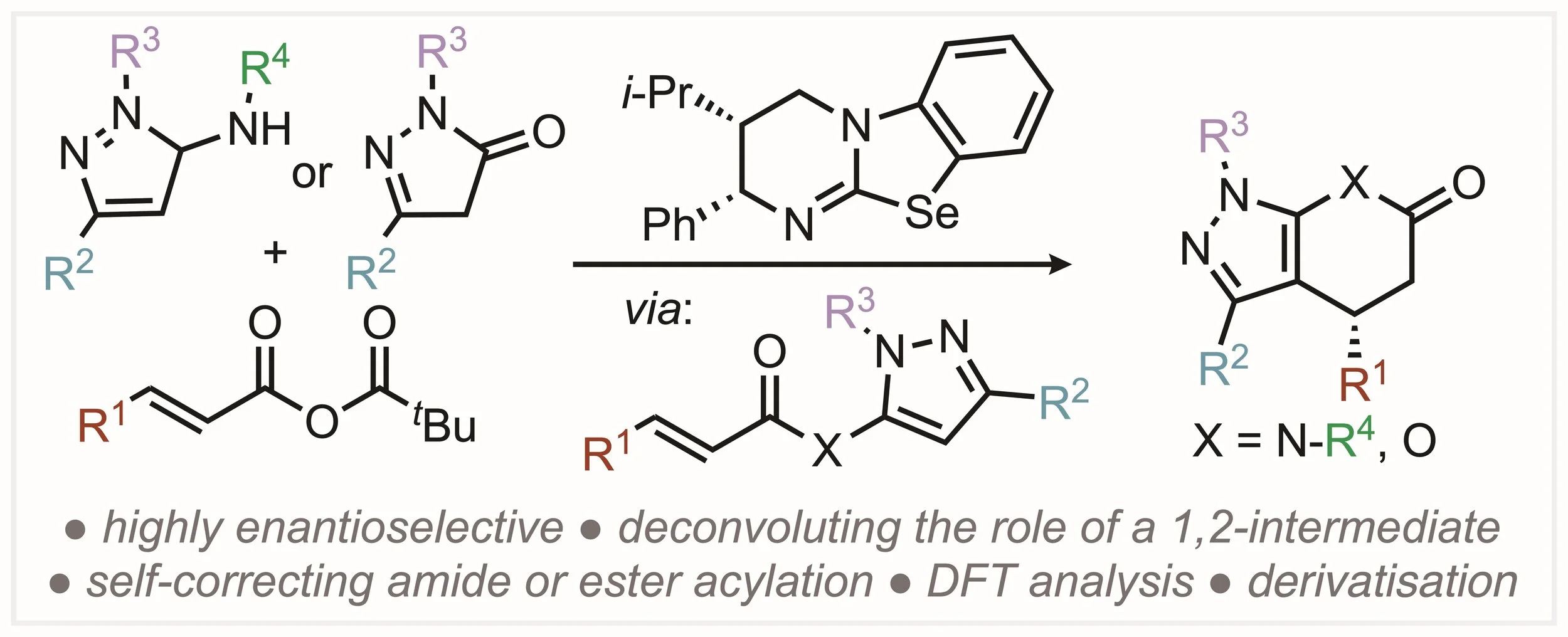
164. Isoselenourea-Catalyzed Enantioselective Pyrazolo-heterocycle Synthesis Enabled By Self-correcting Amide and Ester Acylation
M. I. Prindl, M. T. Westwood, A. S. Goodfellow, A. P. McKay, D. B. Cordes, M. Bühl and A. D. Smith
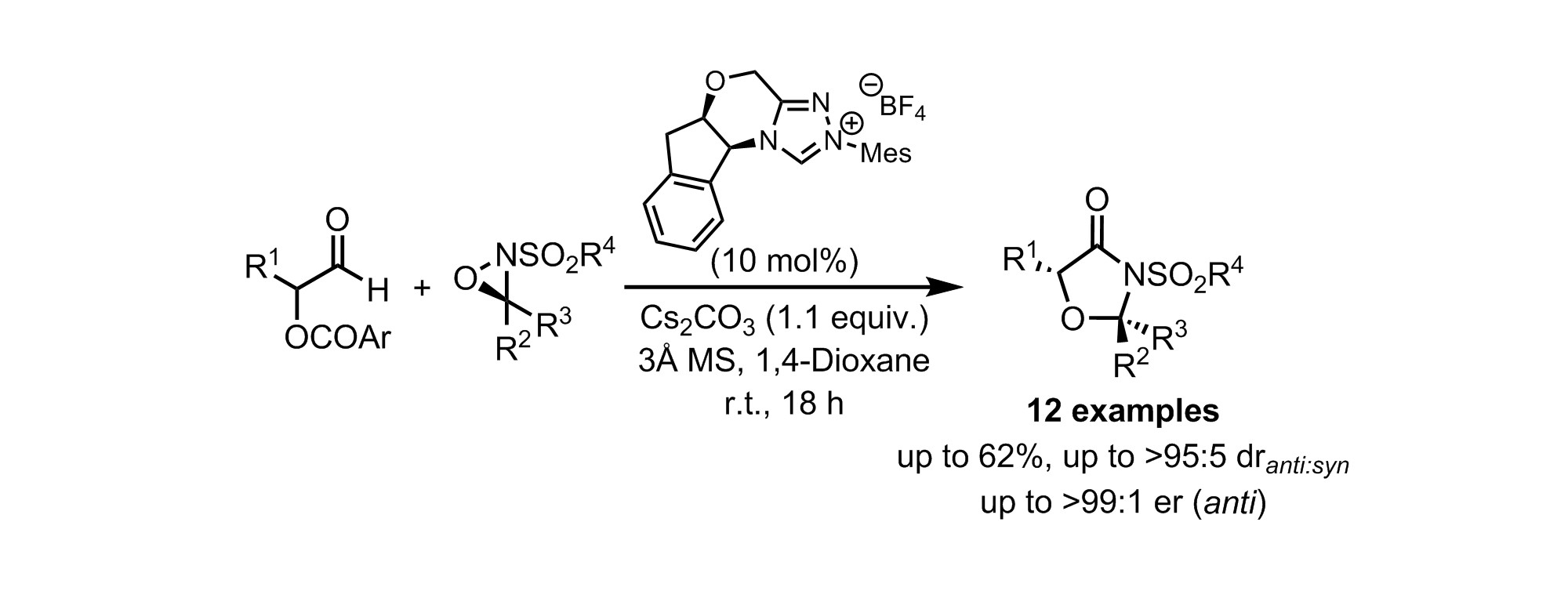
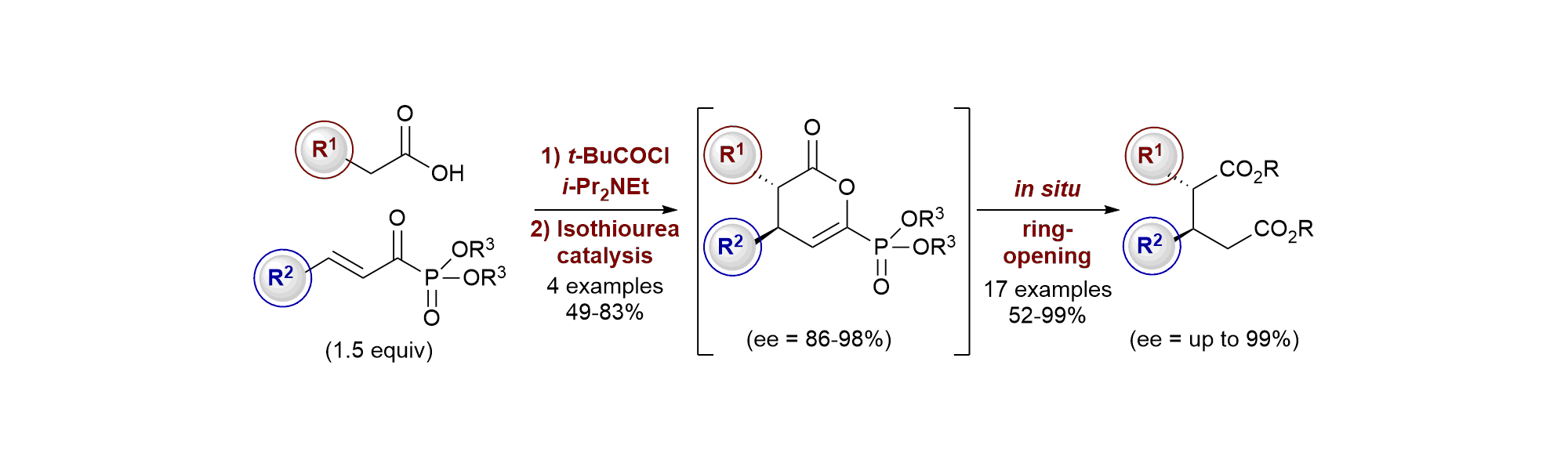
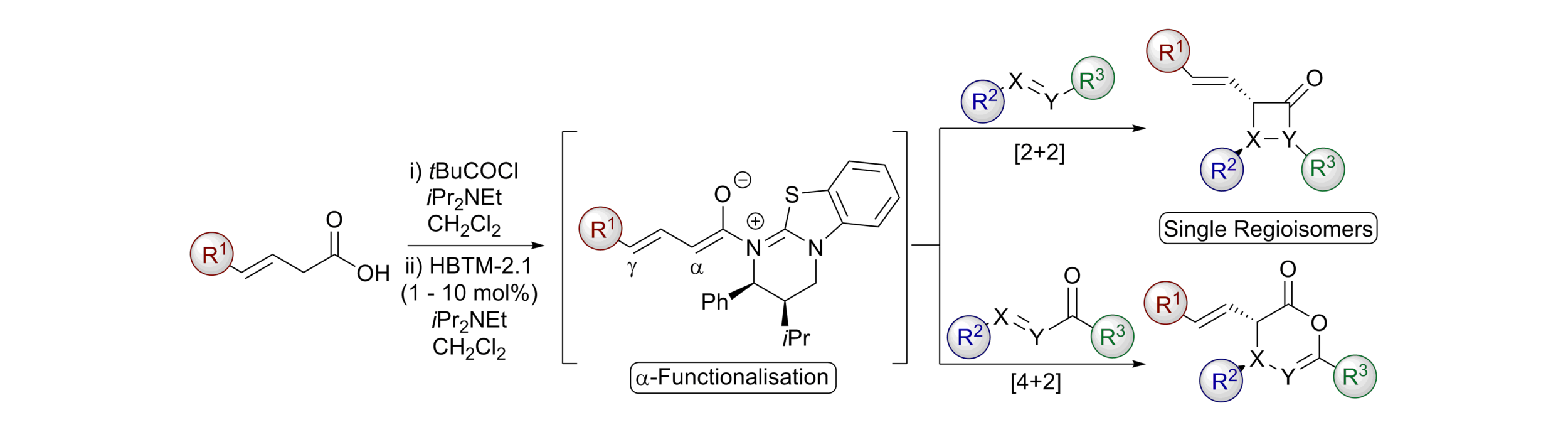
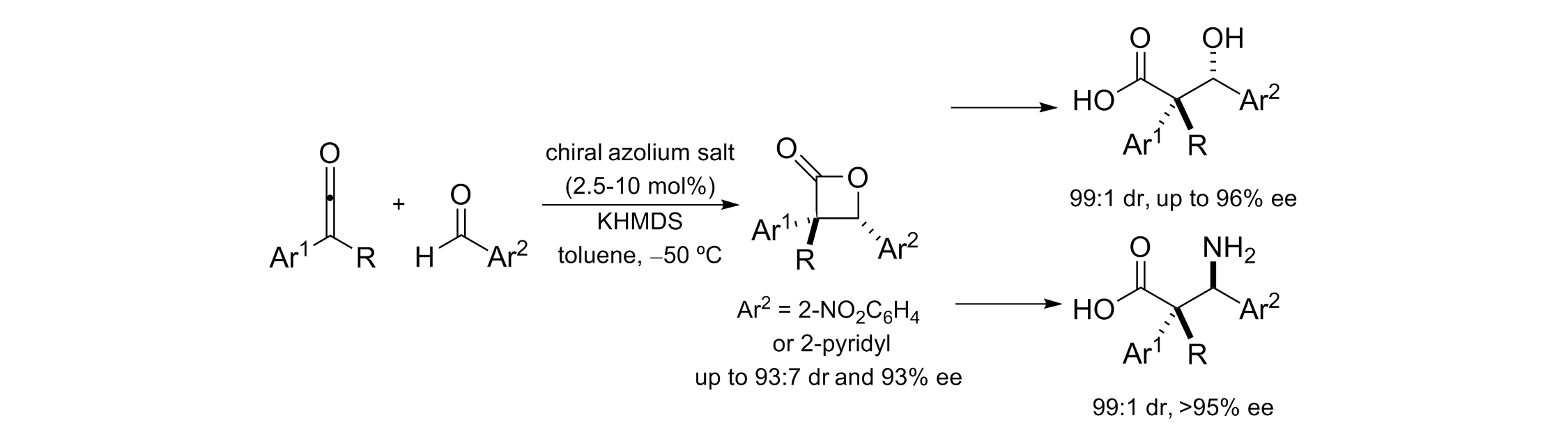
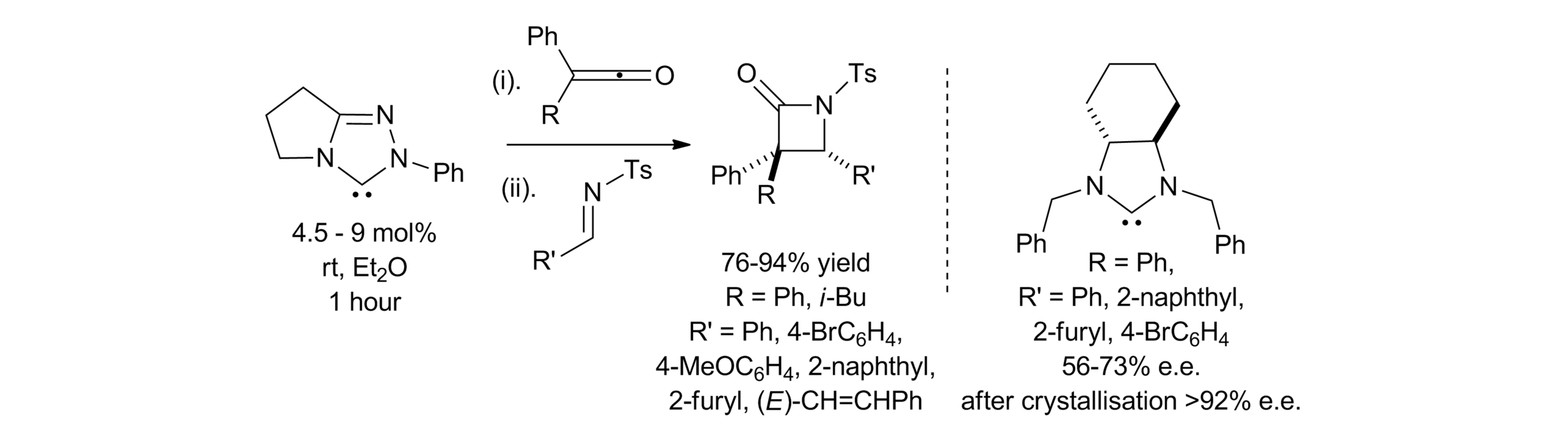
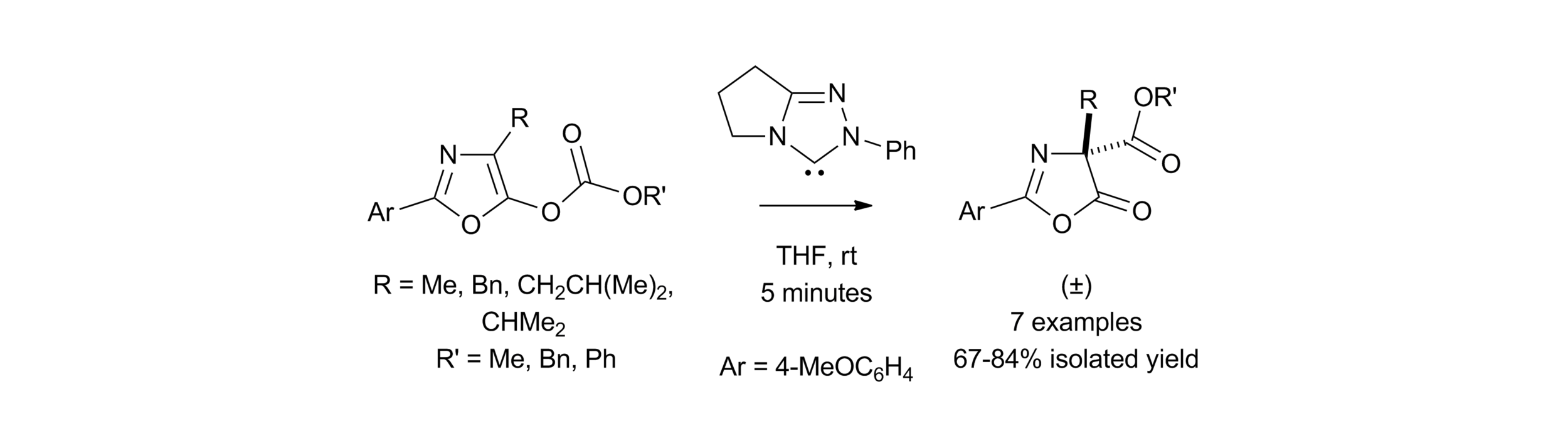
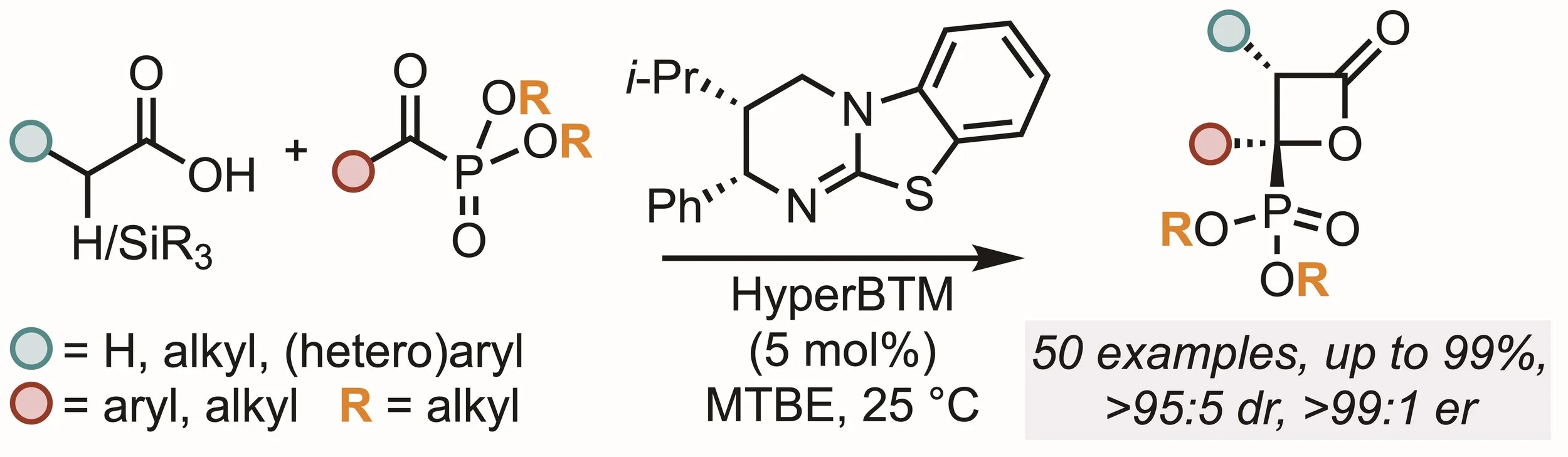
163. Isothiourea-Catalysed Enantioselective Synthesis of Phosphonate-Functionalised β-lactones
F. M. Platt, Y. Wang, D. B. Cordes, A. P. McKay, A. M. Z. Slawin, H. Panchal and A. D. Smith*
162. In-Cage Recombination Facilitates the Enantioselective Organocatalytic [1,2]-Rearrangement of Allylic Ammonium Ylides
W. C. Hartley, K. Kasten, M. D. Greenhalgh, T. Feoktistova, H. R. Wise, J. M. Laddusaw, A. B. Frost, S. Ng, A. M. Z. Slawin, B. E. Bode, P. Ha-Yeon Cheong*, A. D. Smith*
161. Isothiourea-Catalysed Acylative Kinetic Resolution of Tertiary Pyrazolone Alcohols
M. T. Westwood, A. O. Farah, H. B. Wise, M. Sinfield, C. Robichon, D. B. Cordes, P. H-Y. Cheong, A. D. Smith
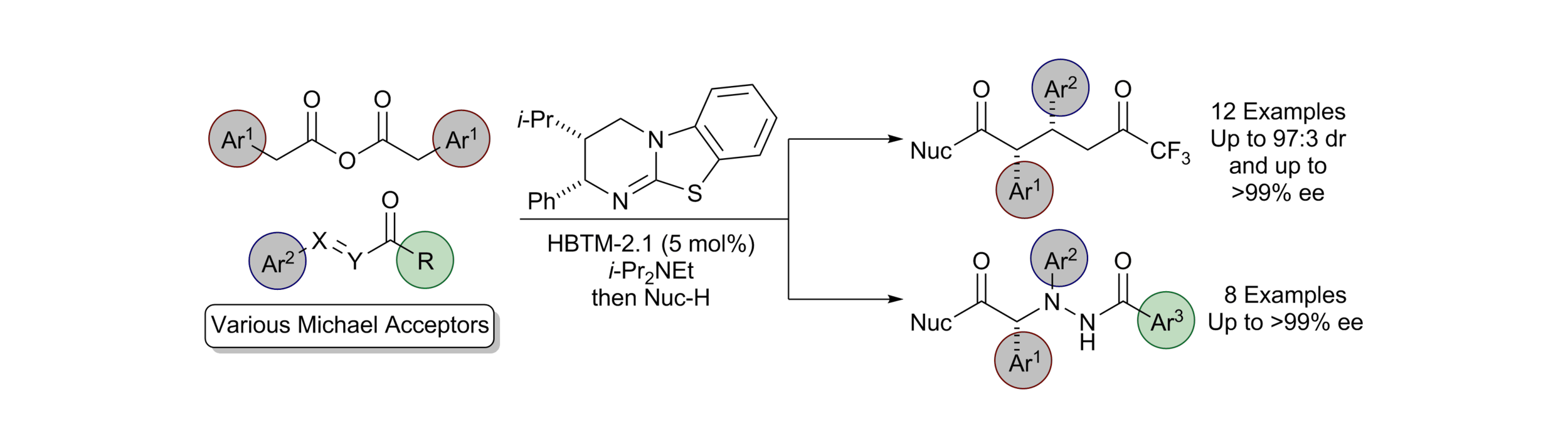
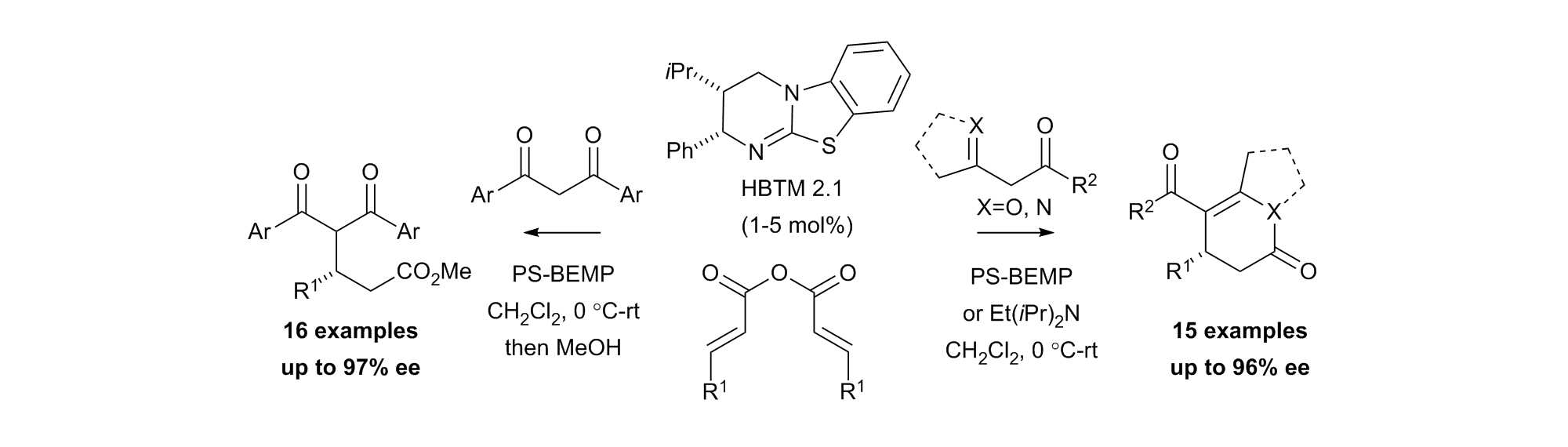
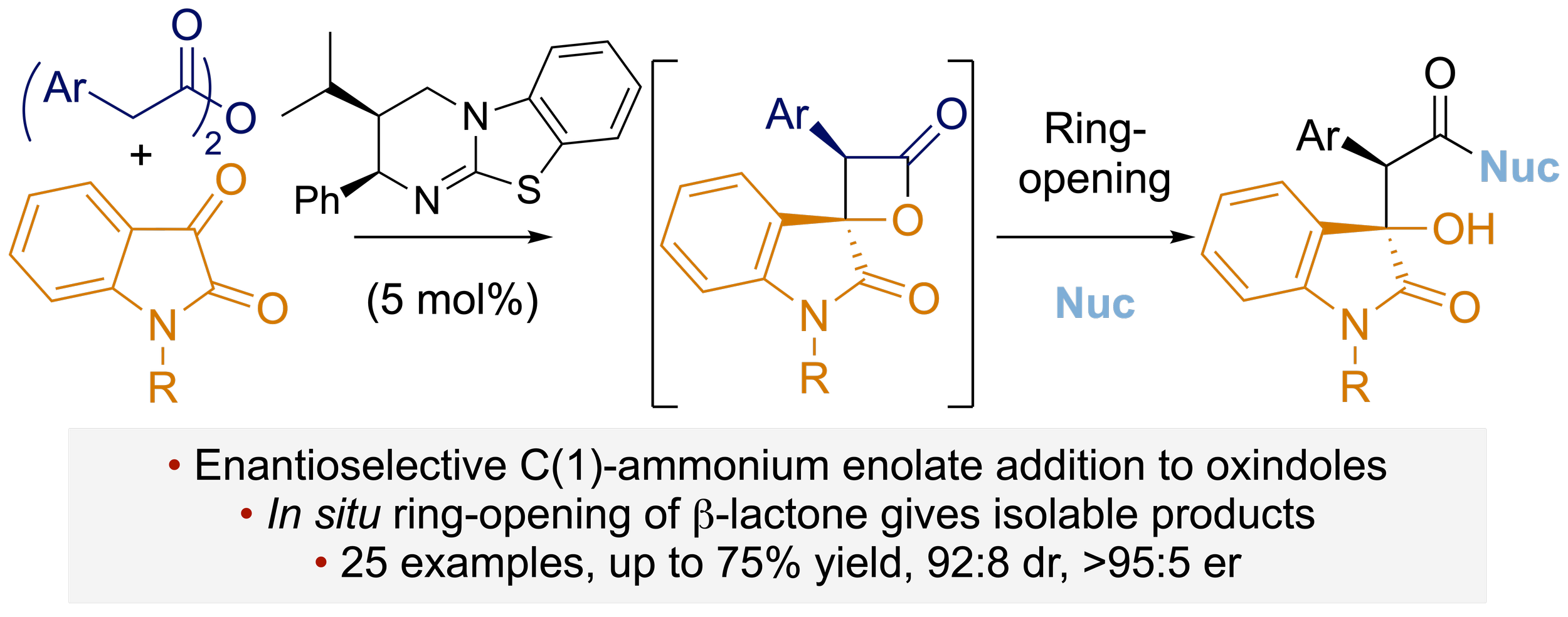
160. One-Pot Access to Functionalised Malamides via Organocatalytic Enantioselective Formation of Spirocyclic β-Lactone-Oxindoles and Double Ring-Opening
A. J. Nimmo, K. Kasten, G. White, J. Roeterdink, A. P. McKay, D. B. Cordes, A. D. Smith
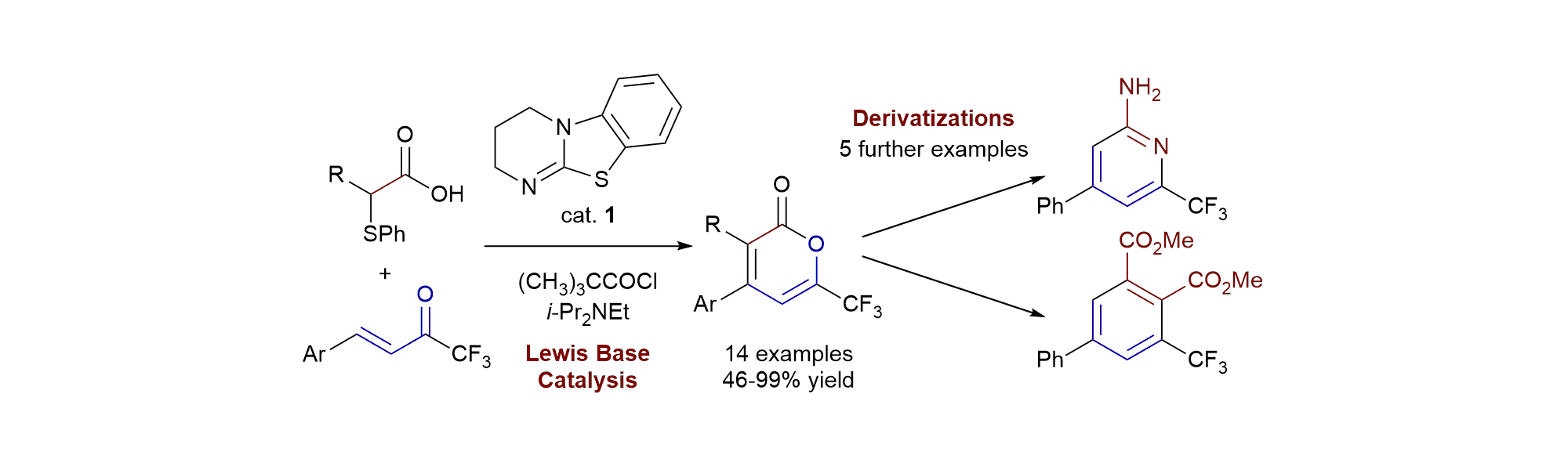
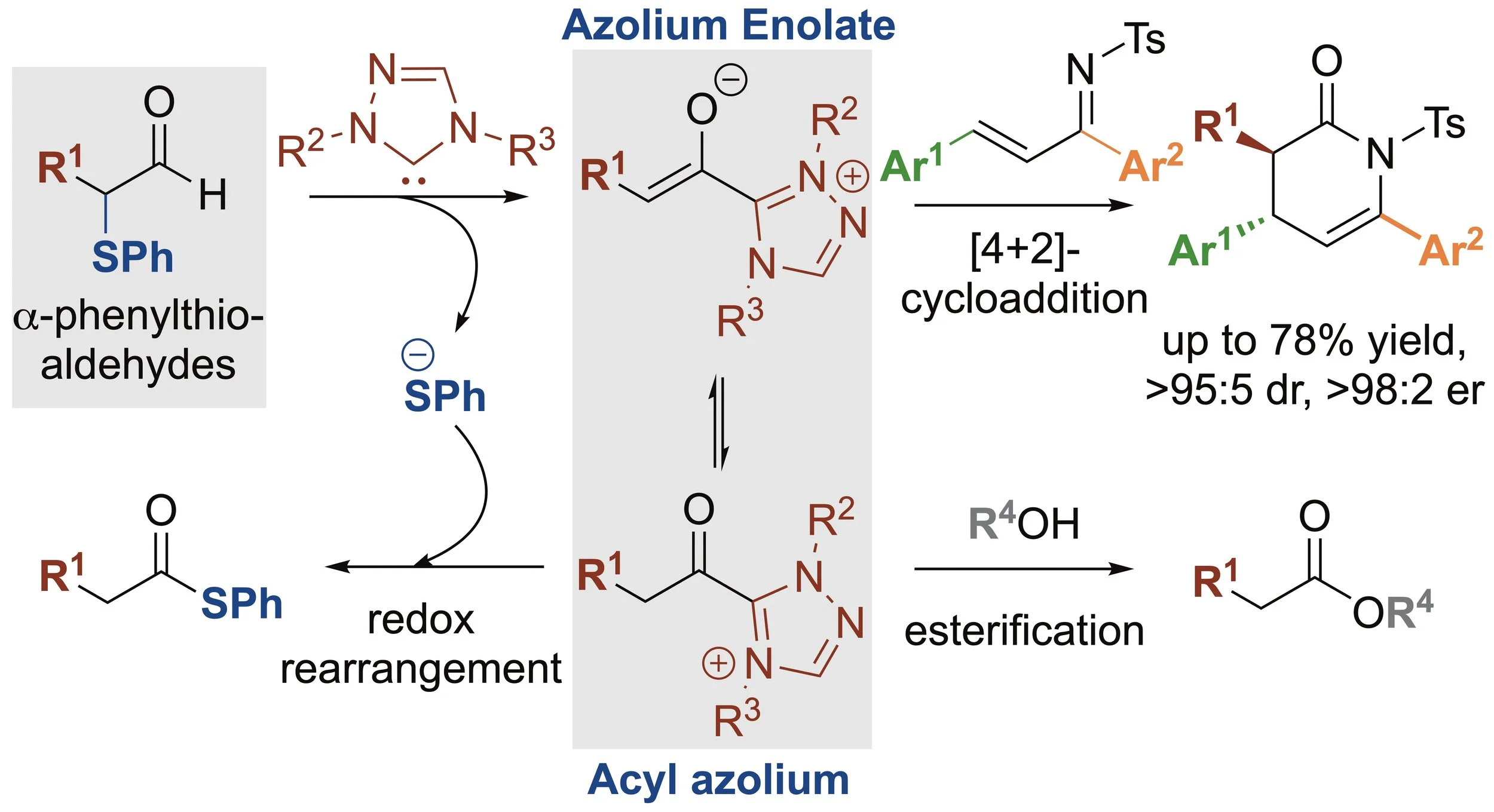
159. a-Phenylthioaldehydes for the effective generation of acyl azolium and azolium enolate intermediates
P. M. D. A. Ewing, P. K. Majhi, C. Prentice, C. M. Young, K. van Rees, P. L. Arnold, E. Zysman-Colman, A. D. Smith
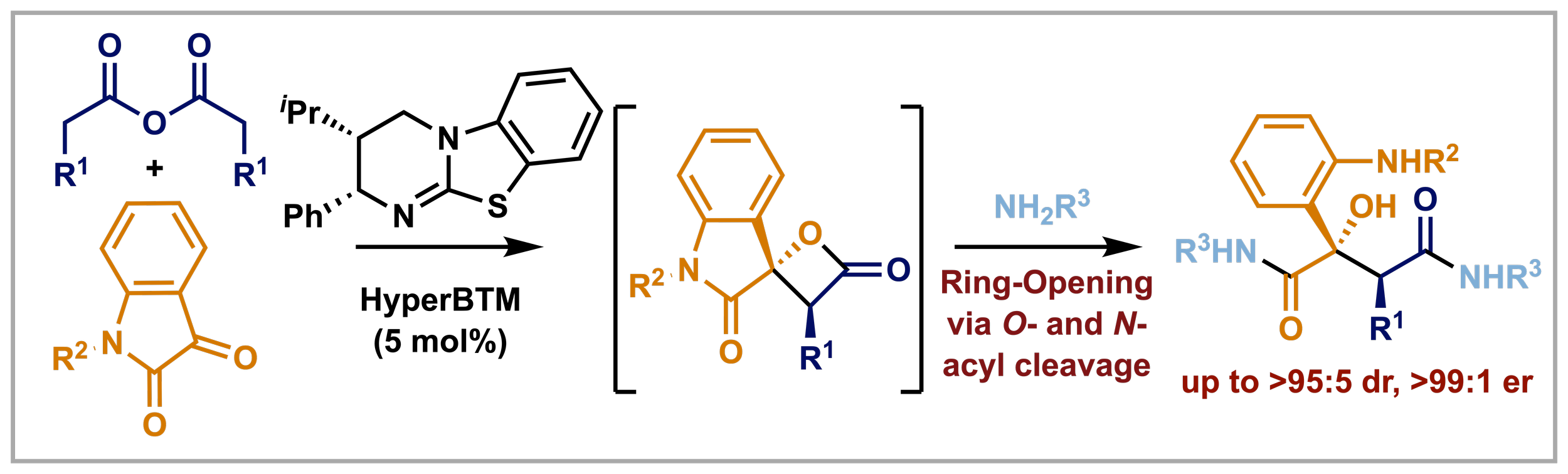
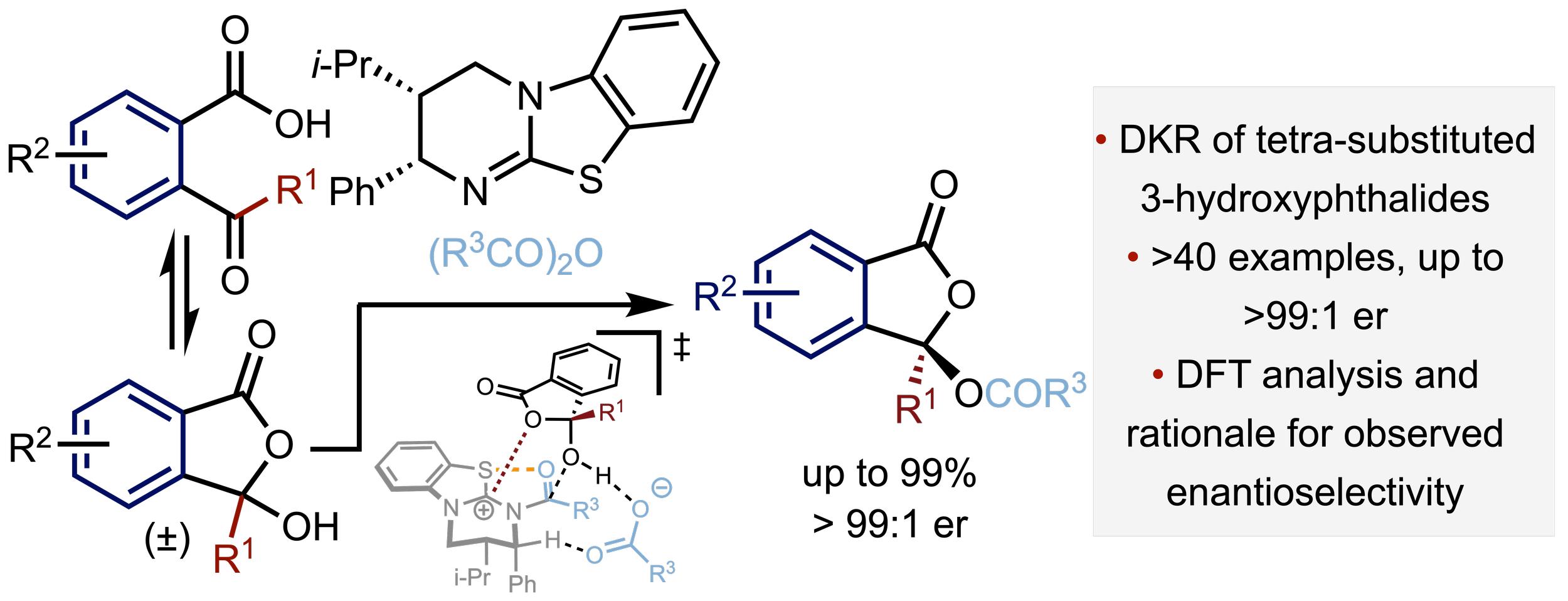
158. Enantioselective Synthesis of Tetra-substituted 3-Hydroxyphthalide Esters by Isothiourea-Catalysed Acylative Dynamic Kinetic Resolution
S. K. Agrawal, P. K. Majhi, A. S. Goodfellow, R. K. Tak, David B. Cordes, Aidan P. McKay, Kevin Kasten, Michael Bühl, A. D. Smith
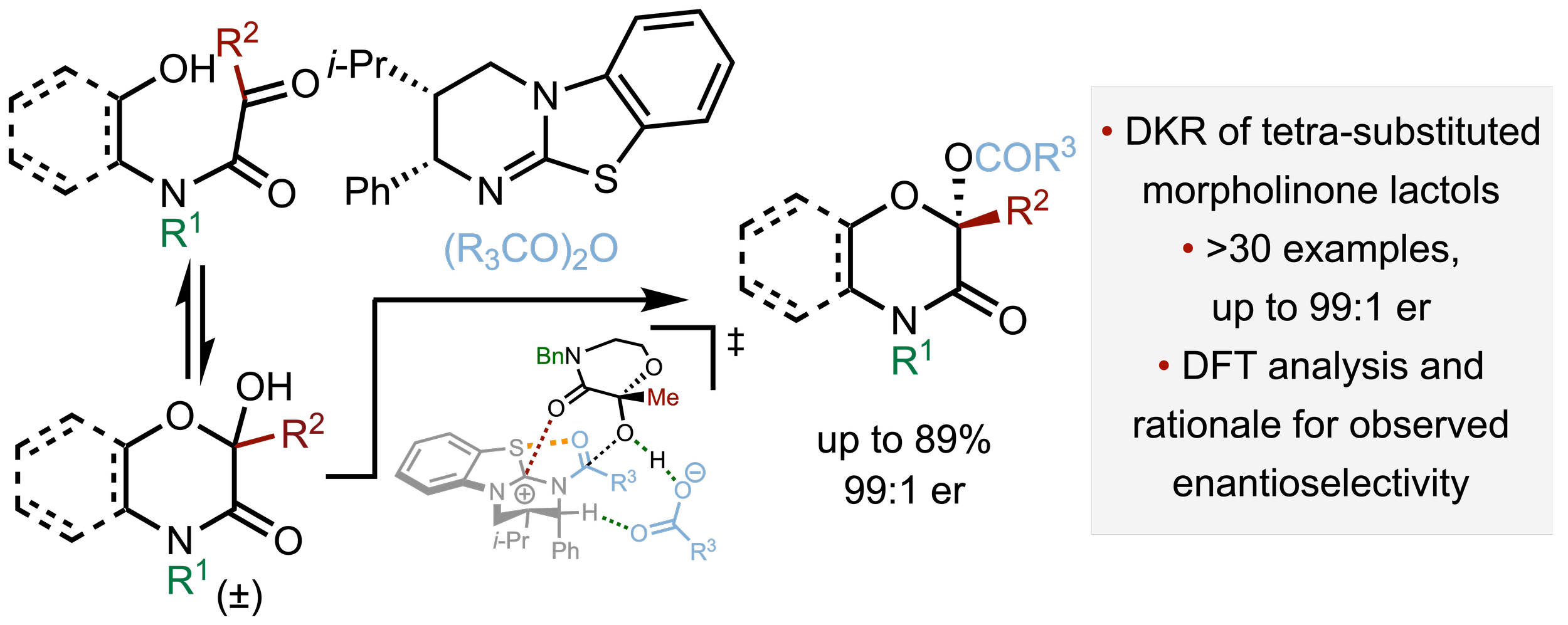
157. Isothiourea-Catalysed Acylative Dynamic Kinetic Resolution of Tetra-substituted Morpholinone and Benzoxazinone Lactols
H. Zhu, A. Manchado, A. O. Farah, A. P. McKay, D. B. Cordes, P. H-Y. Cheong, K. Kasten, A. D. Smith
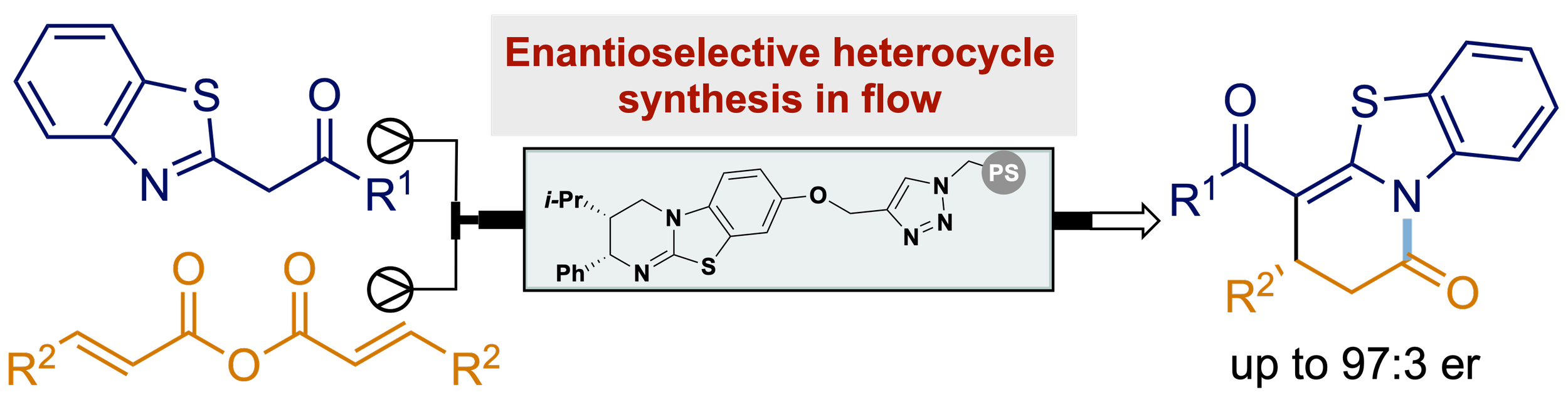
156. Enantioselective Synthesis in Continuous Flow: Polymer Supported Isothiourea Catalyzed Enantioselective Michael Addition-Cyclisation with α-Azol-2-ylacetophenones
Z. Zhou, K. Kasten, T. Kang, D. B. Cordes, A. D. Smith
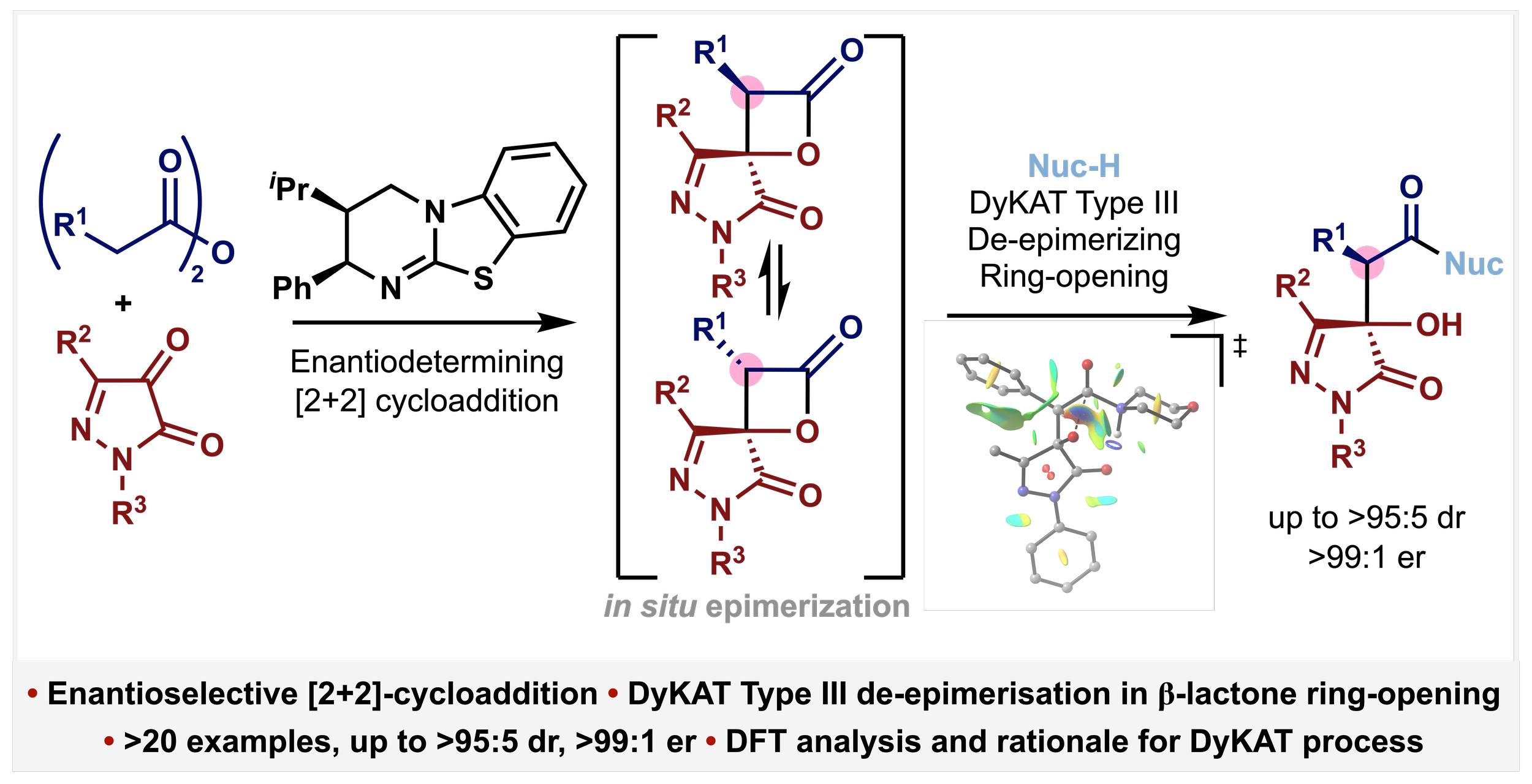
155. De-epimerizing DyKAT of β-Lactones Generated by Isothiourea-Catalysed Enantioselective [2+2] Cycloaddition
A. Conboy, A. S. Goodfellow, K. Kasten, J. Dunne, D. B. Cordes, M. Bühl, A. D. Smith
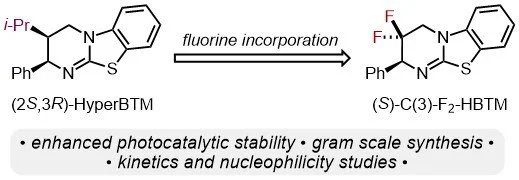
154. Unveiling the impact of a CF2 motif in the isothiourea catalyst skeleton: Evaluating C(3)-F2-HBTM and its catalytic activity
M. T. Westwood, K. Kasten, L. Stockhammer, R. d. Río-Rodríguez, J. A. Fernández-Salas, A. Eitzinger, A. M. Z. Slawin, M. Waser, J. Alémán, A. R. Ofial, A. D. Smith
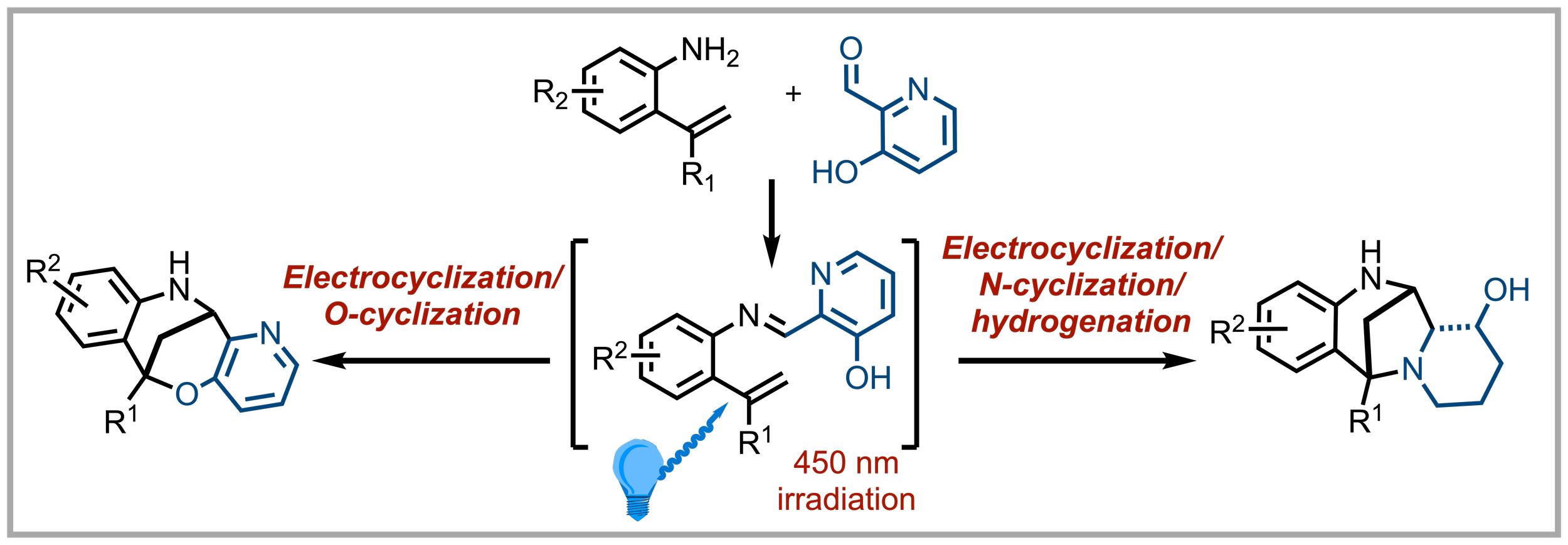
153. Access to a Diverse Array of Bridged Benzo[1,5]oxazocine and Benzo[1,4]diazepine Structures
G. Sherborne, D. Coura, P. Kemmitt, A. D. Smith
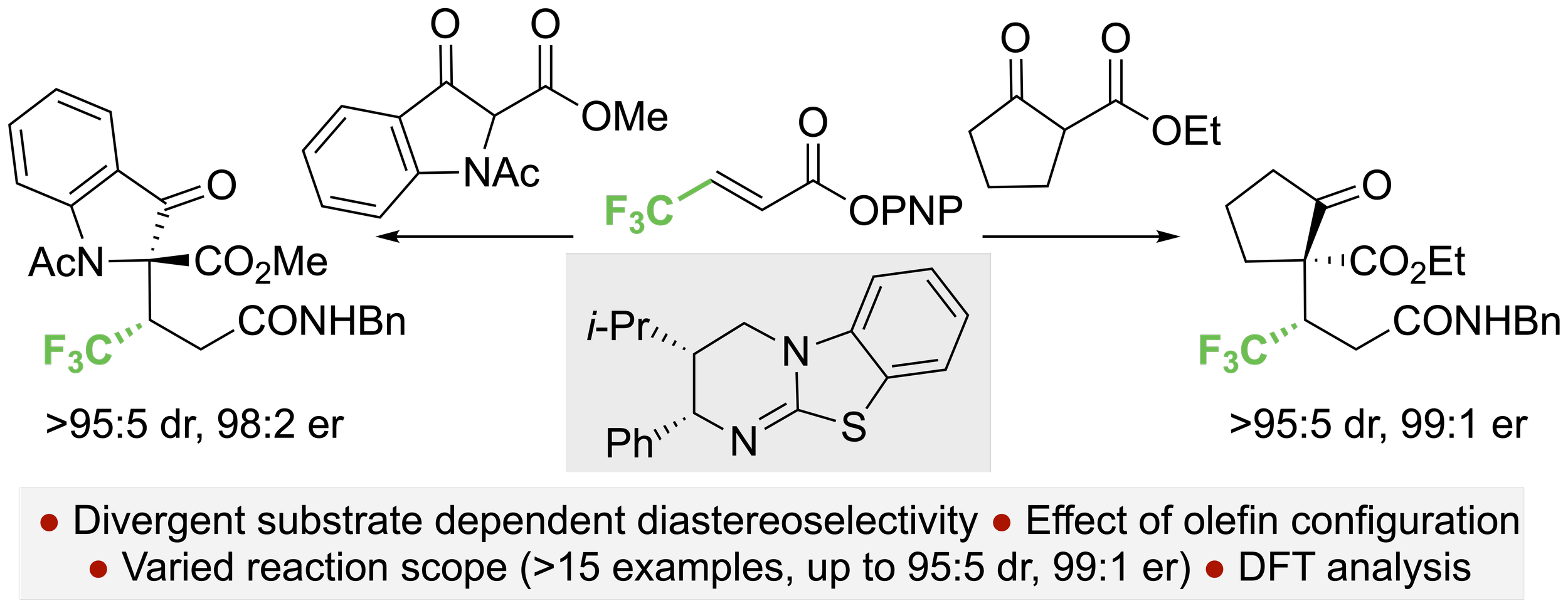
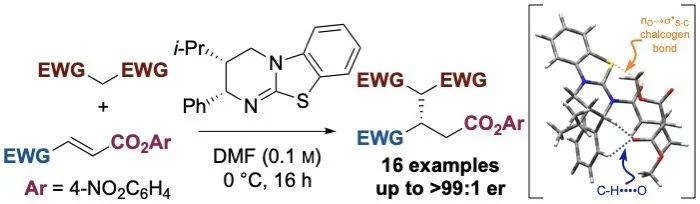
152. Understanding Divergent Substrate Stereoselectivity in the Isothiourea-Catalysed Conjugate Addition of Cyclic a-Substituted b-Ketoesters to a,b-unsaturated Aryl Esters
D. Yuan, A. S. Goodfellow, K. Kasten, Z. Duan, T. Kang, D. B. Cordes, A. P. McKay, M. Bühl, G. R. Boyce, A. D. Smith
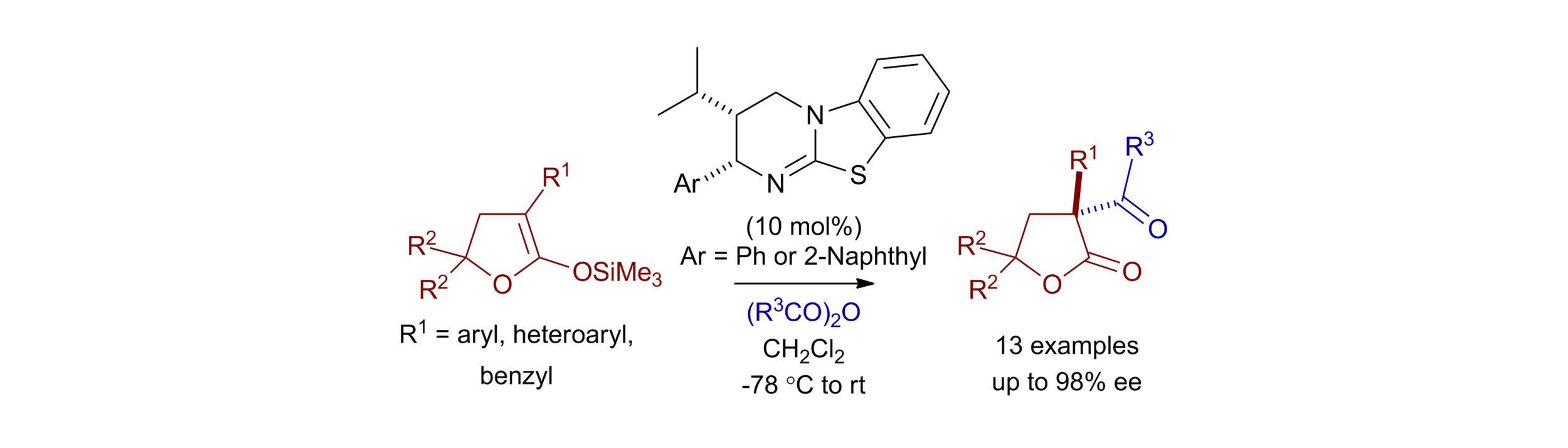
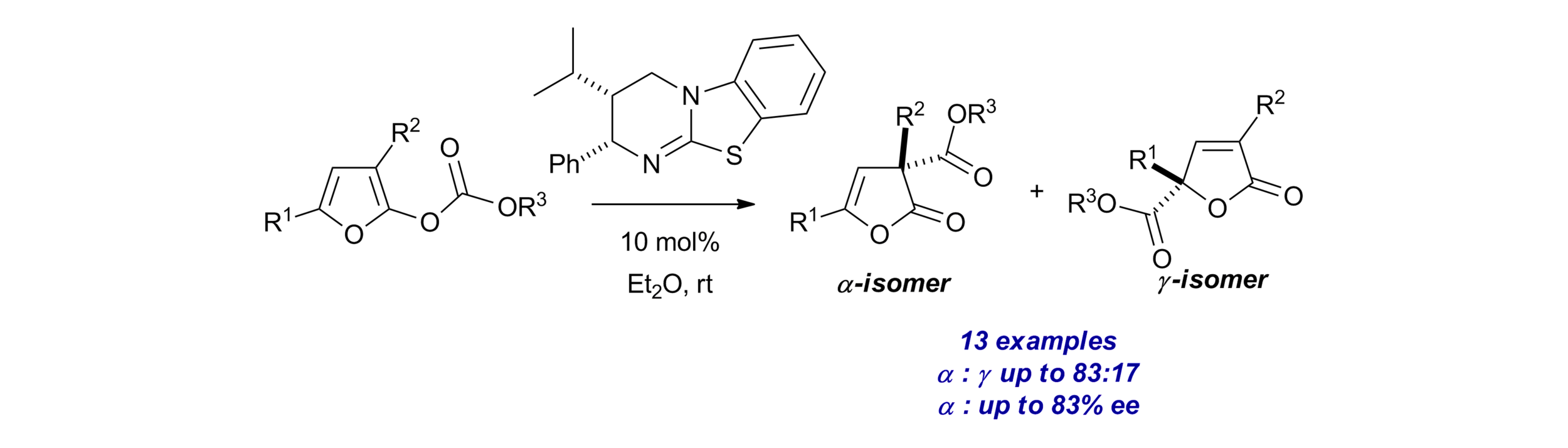
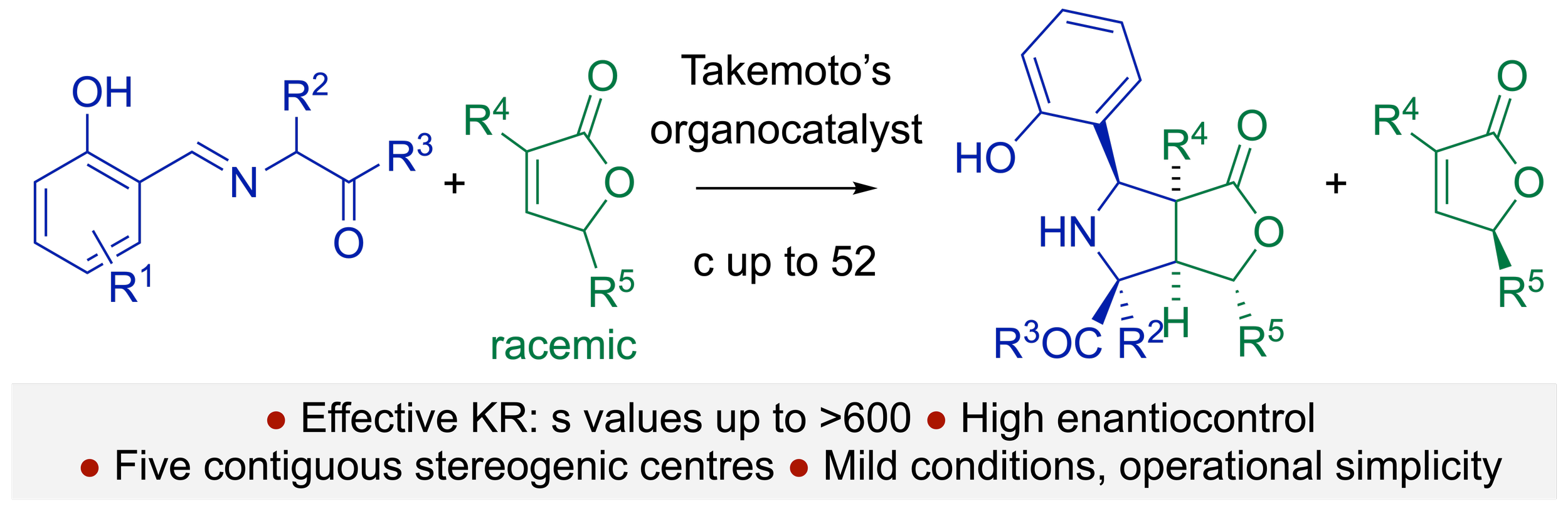
151. Intramolecular Hydrogen Bond Activation for Kinetic Resolution of Furanone Derivatives by an Organocatalyzed [3+2] Asymmetric Cycloaddition
M. A. Valle-Amores, C. Feberero, A. Martin-Somer, S, Díaz-Tendero, A. D. Smith, A. Fraile, J. Alemán
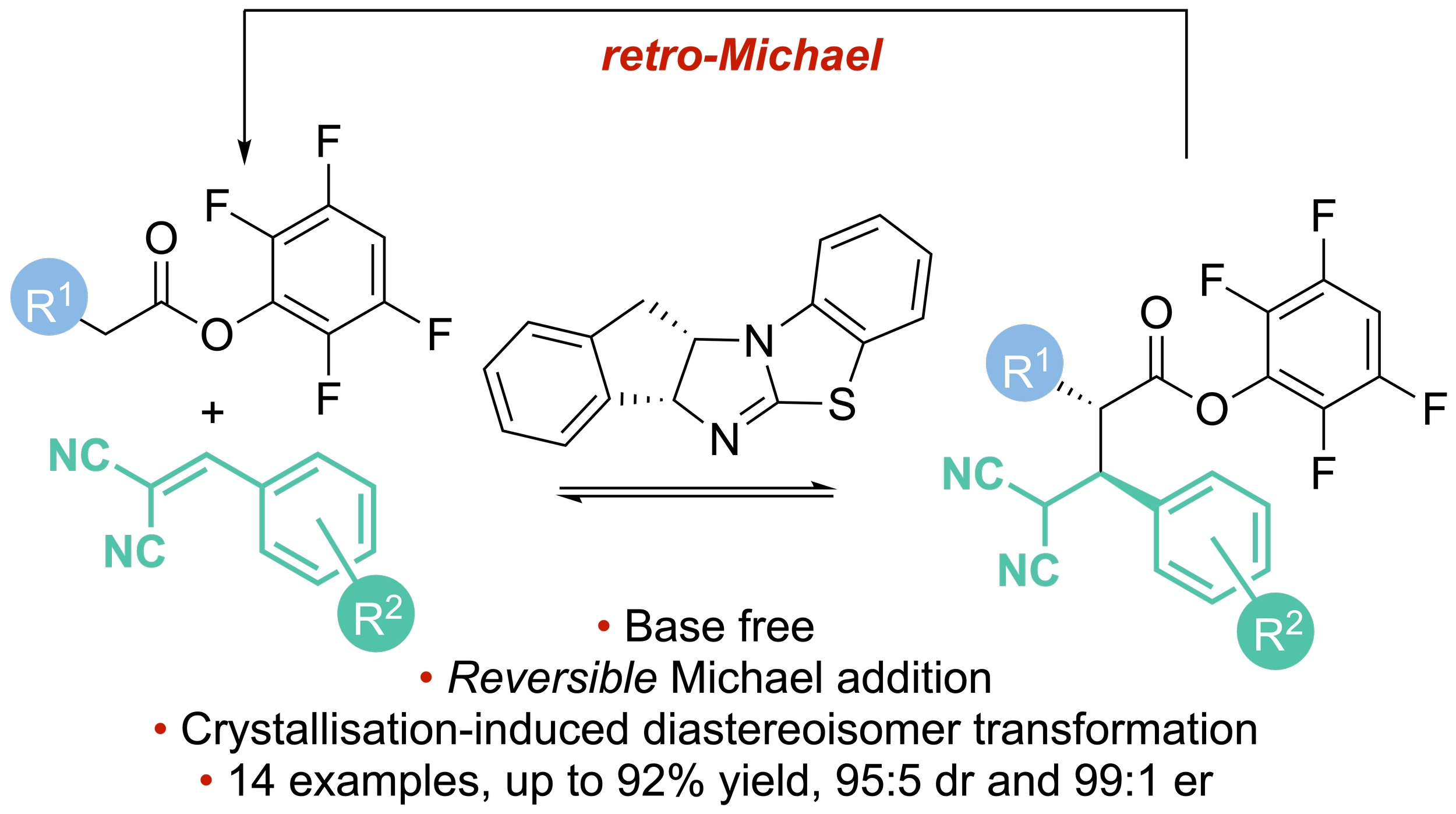
150. Enantioselective isothiourea-catalysed reversible Michael addition of aryl esters to 2-benzylidene malononitriles
Alastair J. Nimmo, Jacqueline Bitai, Claire M. Young, Calum McLaughlin, Alexandra M. Z. Slawin, David B. Cordes and Andrew D. Smith
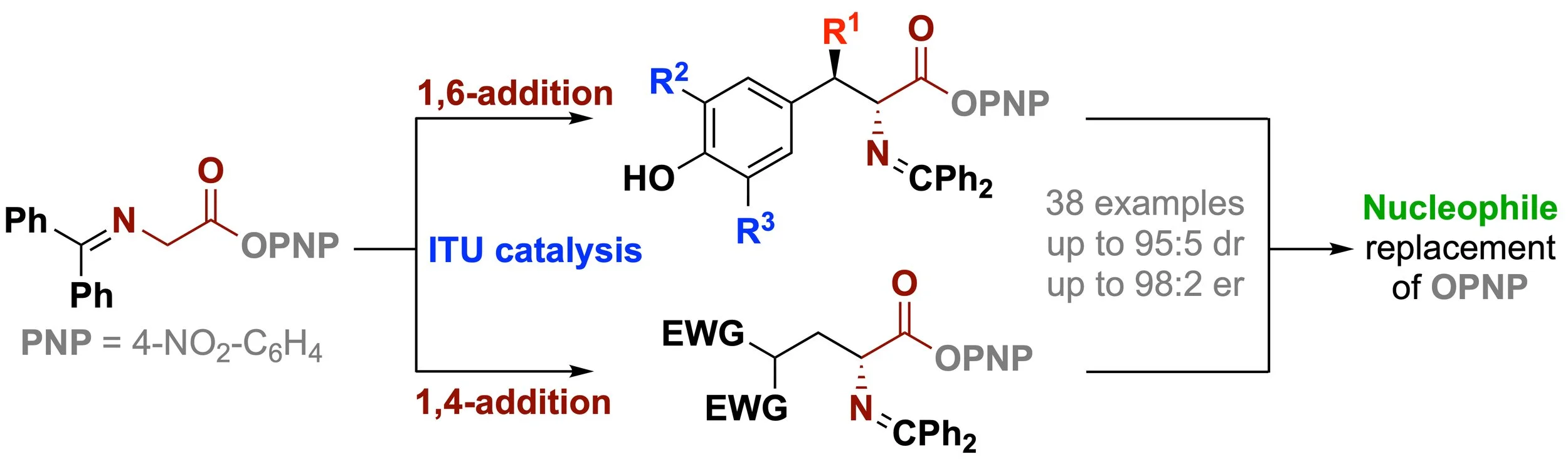
149. Isothiourea-Catalyzed Enantioselective Functionalisation of Glycine Schiff Base Aryl Esters via 1,6- and 1,4-additions
L. Stockhammer, R. Craik, U. Monkowius, D. B. Cordes, A. D. Smith, M. Waser
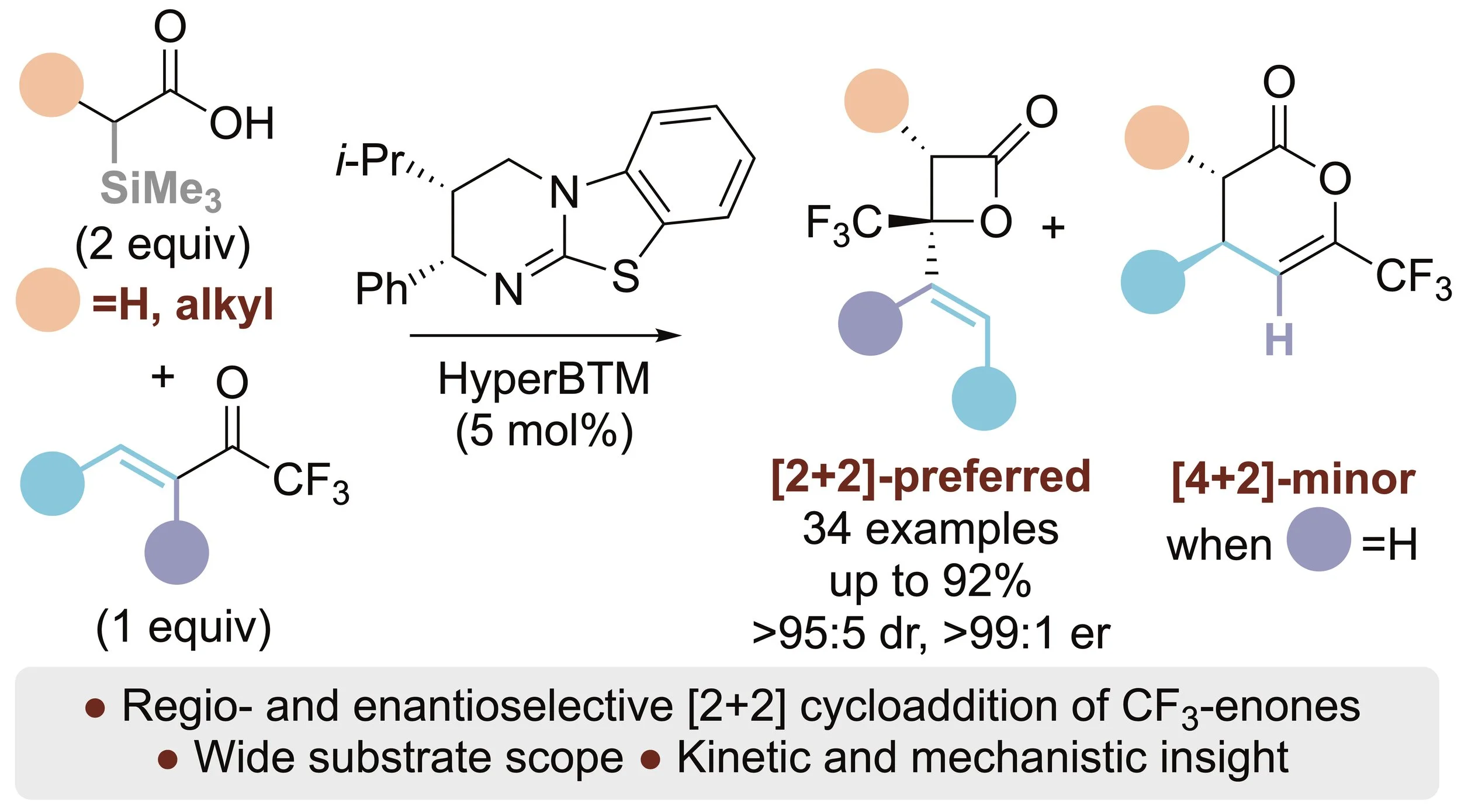
148. Probing Regio- and Enantioselectivity in the Formal [2+2] Cycloaddition of C(1)-Alkyl Ammonium Enolates with β- and α,β-Substituted Trifluoromethylenones
Y. Wang, C. M. Young, D. B. Cordes, A. M. Z. Slawin, A. D. Smith
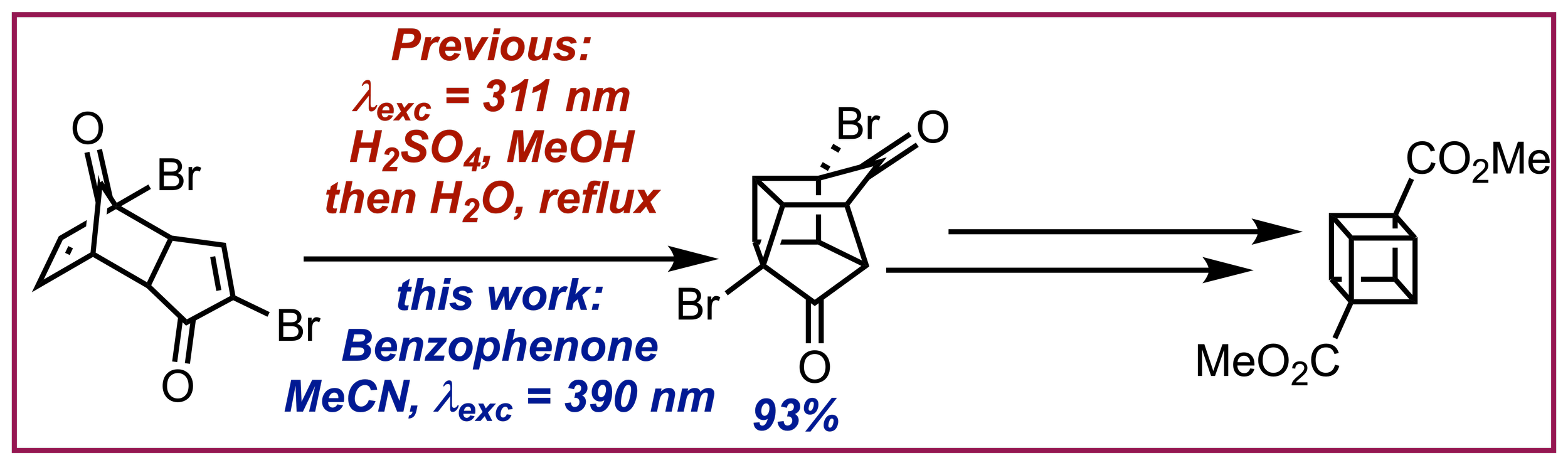
147. Benzophenone as a cheap and effective photosensitizer for the photocatalytic synthesis of dimethyl cubane-1,4-dicarboxylate
C. Prentice, A. E. Martin, J. Morrison, A. D. Smith, E. Zysman-Colman
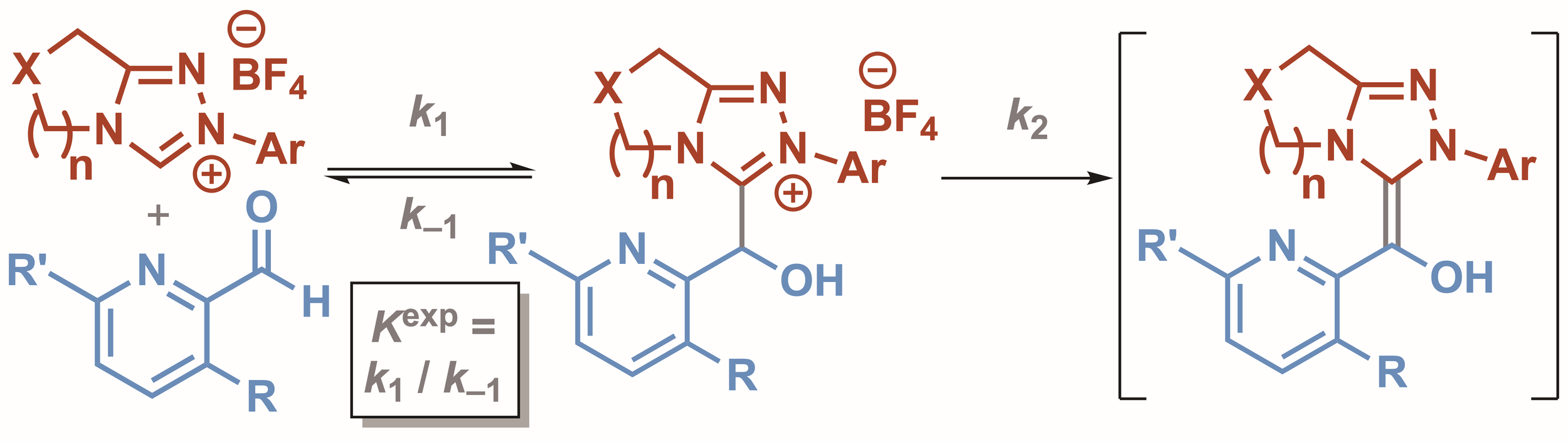
146. Rate and Equilibrium Constants for the Addition of Triazolium Salt Derived N-Heterocyclic Carbenes to Heteroaromatic Aldehydes
Z. Duan, C. M. Young, J. Zhu, A. M. Z. Slawin, A. C. O’Donoghue, A. D. Smith
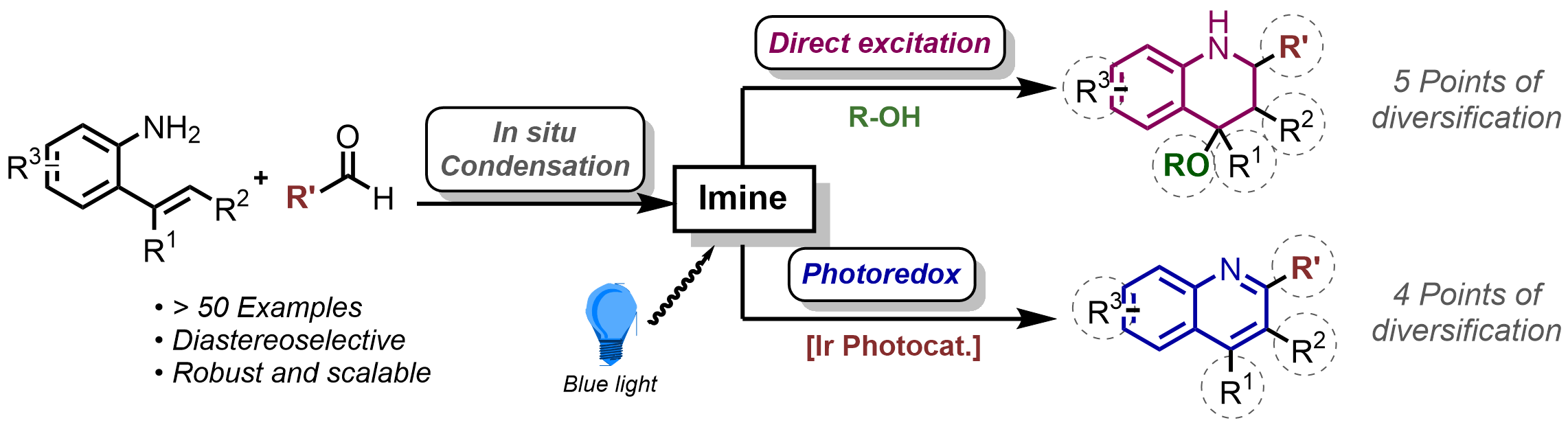
145. Visible Light-Mediated Cyclisation Reaction for the Synthesis of Highly-Substituted Tetrahydroquinolines and Quinolines
G. J. Sherborne, P. Kemmitt, C. Prentice, E. Zysman-Colman, A. D. Smith, C. Fallan
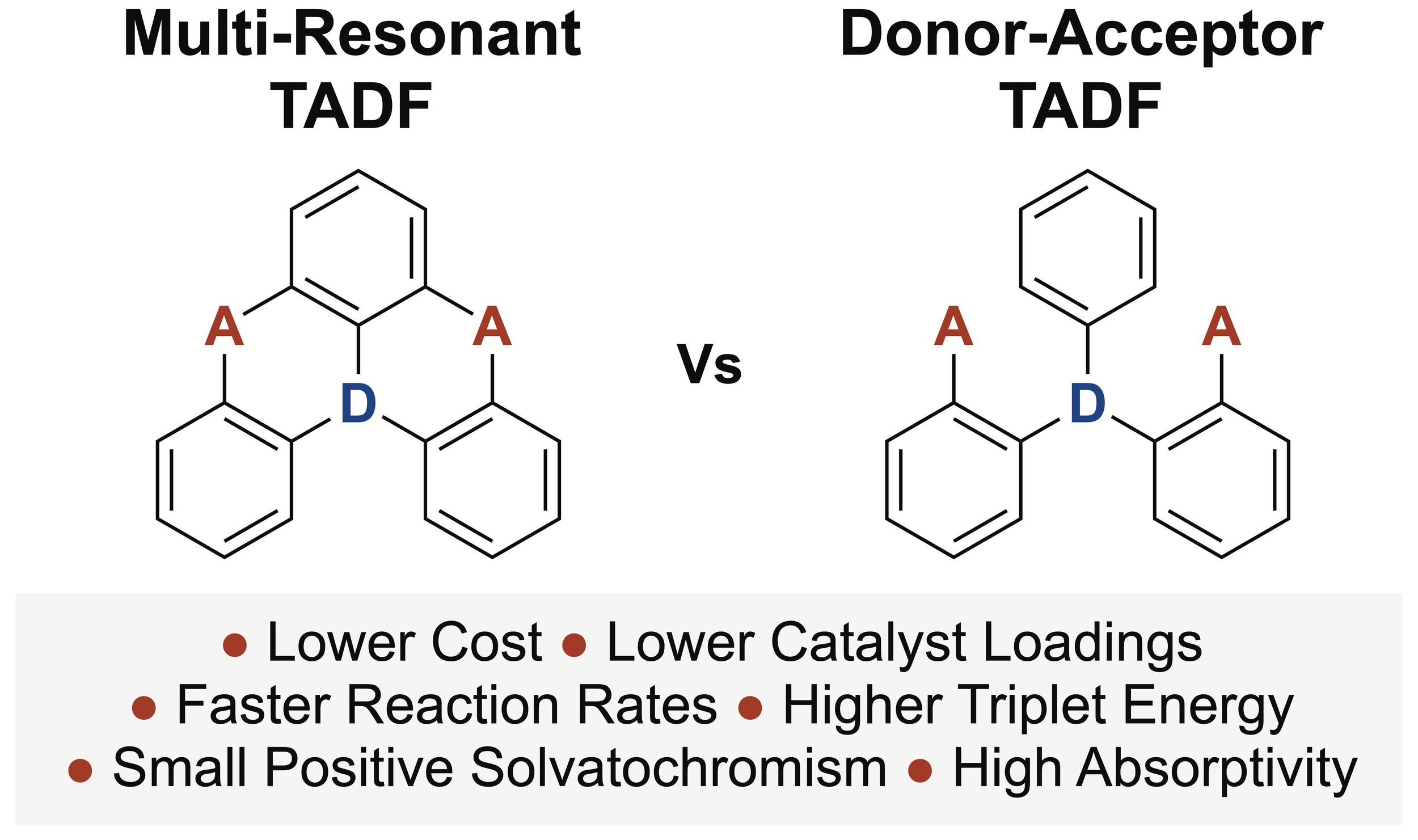
144. Evaluating Multi-Resonant Thermally Activated Delayed (MR-TADF) Fluorescent Compounds as Photocatalysts
C. Prentice, J. Morrison, A. D. Smith, E. Zysman-Colman
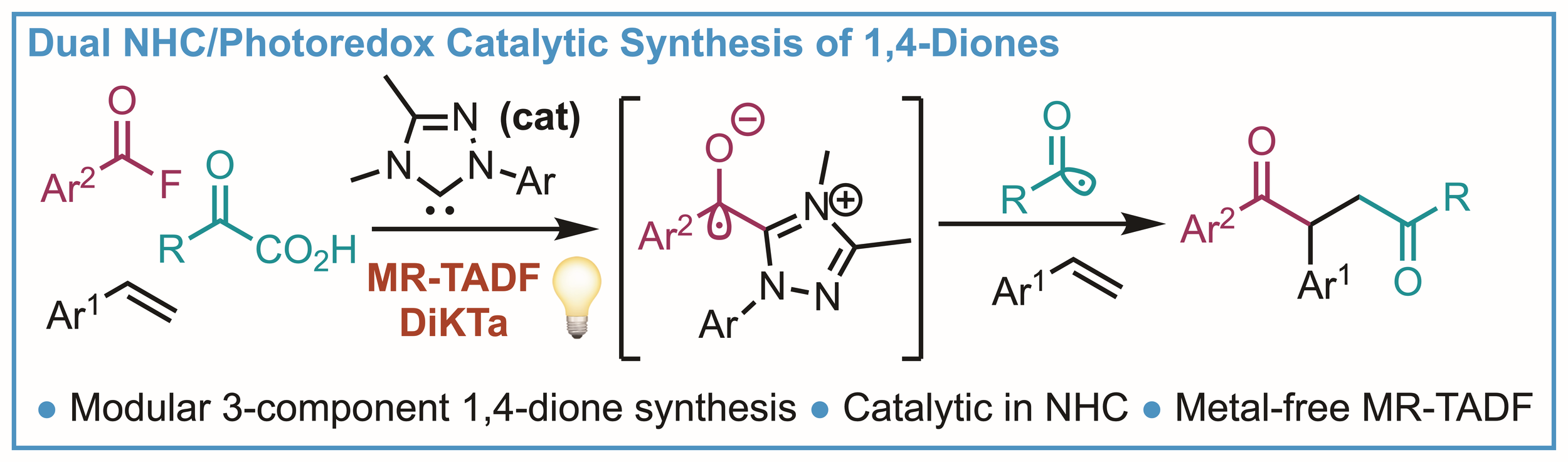
143. Dual NHC/Photoredox Catalytic Synthesis of 1,4-Diketones Using an MR-TADF Photocatalyst (DiKTa)
C. Prentice, J. Morrison, E. Zysman-Colman, A. D. Smith
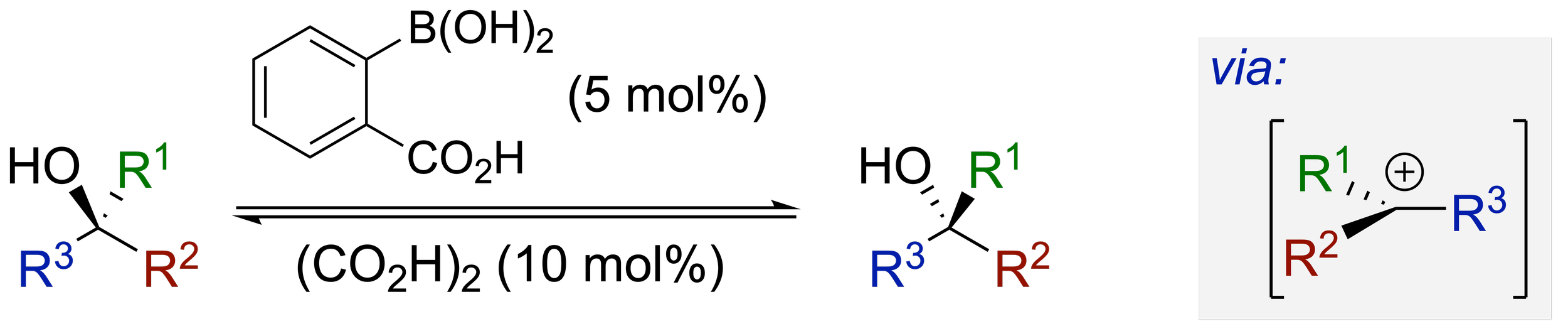
142. Arylboronic Acid-Catalyzed Racemization of Secondary and Tertiary Alcohols
G. R. Boyce, S. F. Musolino, J. Yang, A. D. Smith, J. E. Taylor
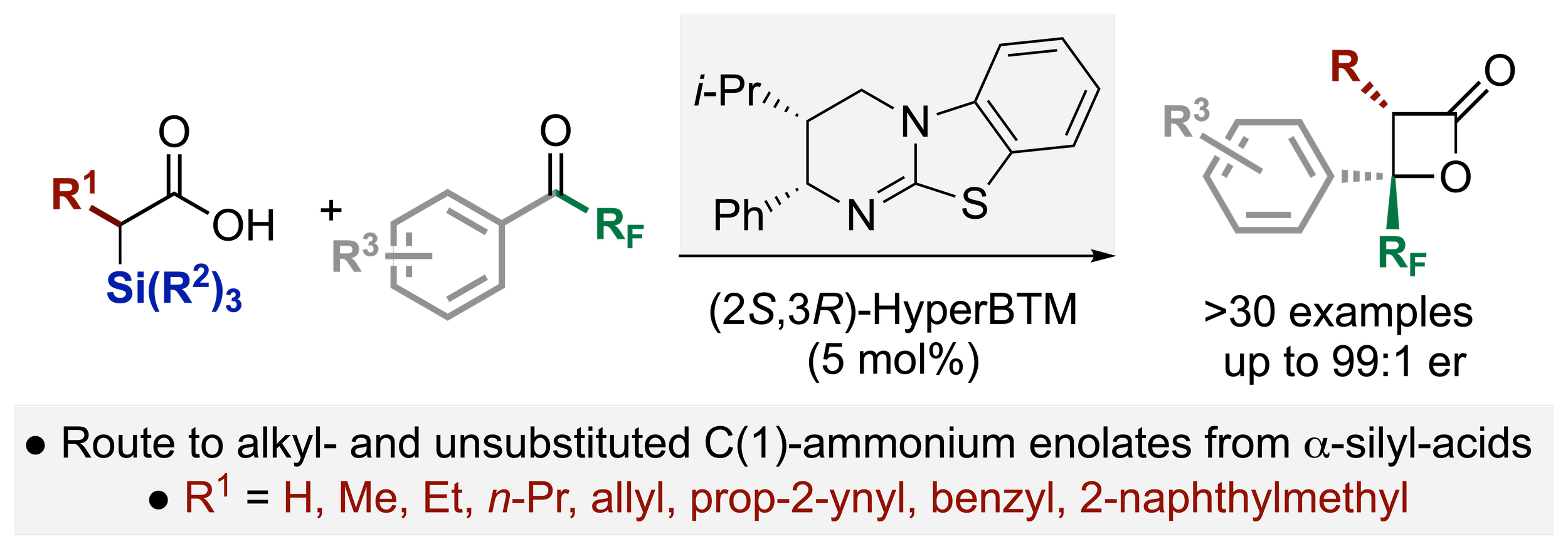
141. A Desilylative Approach to Alkyl Substituted C(1)-Ammonium Enolates: Application In Enantioselective [2+2] Cycloadditions
Y. Wang, C. M. Young, H. Liu, W. C. Hartley, M. Wienhold, D. B. Cordes, A M. Z. Slawin, A. D. Smith
140. Isothiourea-Catalyzed [2+2] Cycloaddition of C(1)-Ammonium Enolates and N-Alkyl Isatins
Y. Abdelhamid, K. Kasten, J. Dunne, W. C. Hartley, C. M. Young, D. B. Cordes, A. M. Z. Slawin, Sean Ng, A. D. Smith
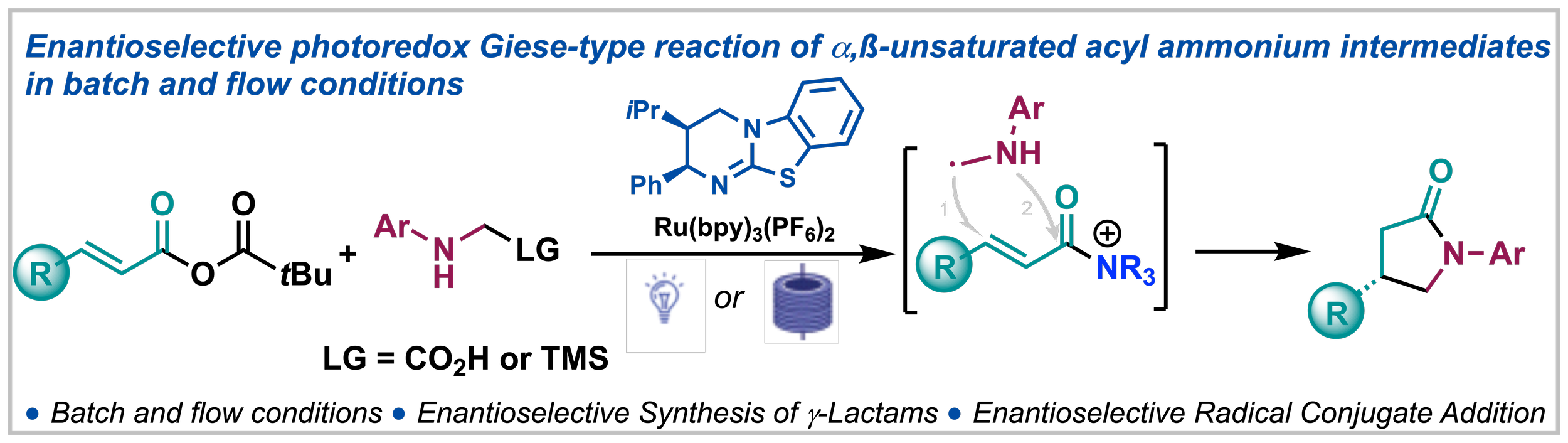
139. Isothiourea-Catalysed Enantioselective Radical Conjugate Addition under Batch and Flow Conditions
R. del Río-Rodríguez, M. T. Westwood, M. Sicignano, M. Juhl, J. A. Fernández-Salas, J. Alemán, A. D. Smith
138. Isothiourea-catalyzed enantioselective Michael addition of malonates to α,β-unsaturated aryl esters
J. Wu, C. M. Young, A. Watts, A. M. Z. Slawin, G. Boyce, M. Bühl, A. D. Smith
137. Isothiourea-Catalyzed Formal Enantioselective Conjugate Addition of Benzophenone Imines to β-Fluorinated α,β-Unsaturated Esters
J. Lapetaje, C. M. Young, C. Shu, A. D. Smith
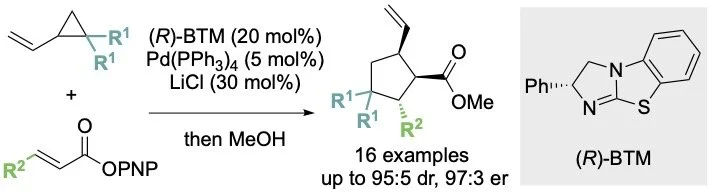
136. Cooperative Palladium/Isothiourea Catalyzed Enantioselective Formal (3+2) Cycloaddition of Vinylcyclopropanes and a,b-Unsaturated Esters
J. Bitai, A. Nimmo, A. M. Z. Slawin and A. D. Smith
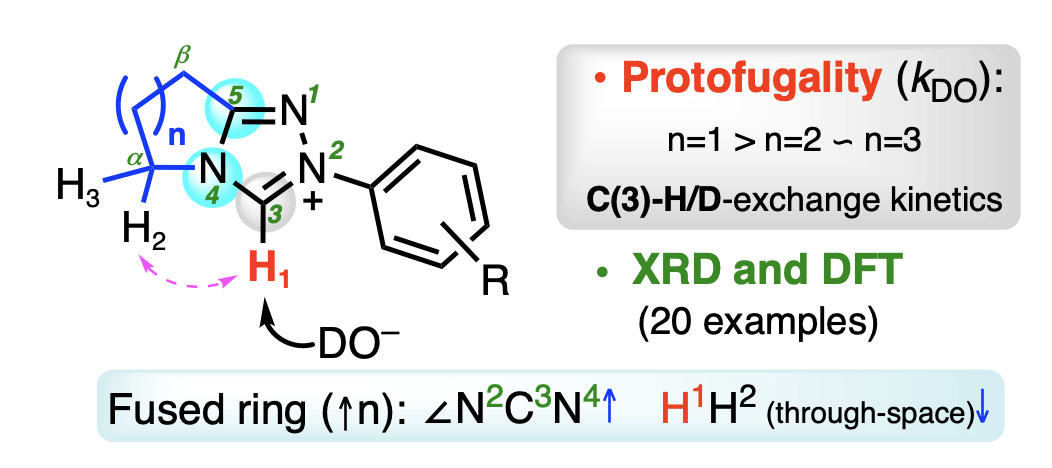
135. The Role of the Fused Ring in Bicyclic Triazolium Organocatalysts: Kinetic, X-ray and DFT Insights
J. Zhu, I. Moreno, P. Quinn, D. Yufit, L. Song, C. M. Young, Z. Duan, A. Tyler, P. Waddell, M. Hall, M. R. Probert, A. D. Smith, A. C. O'Donoghue
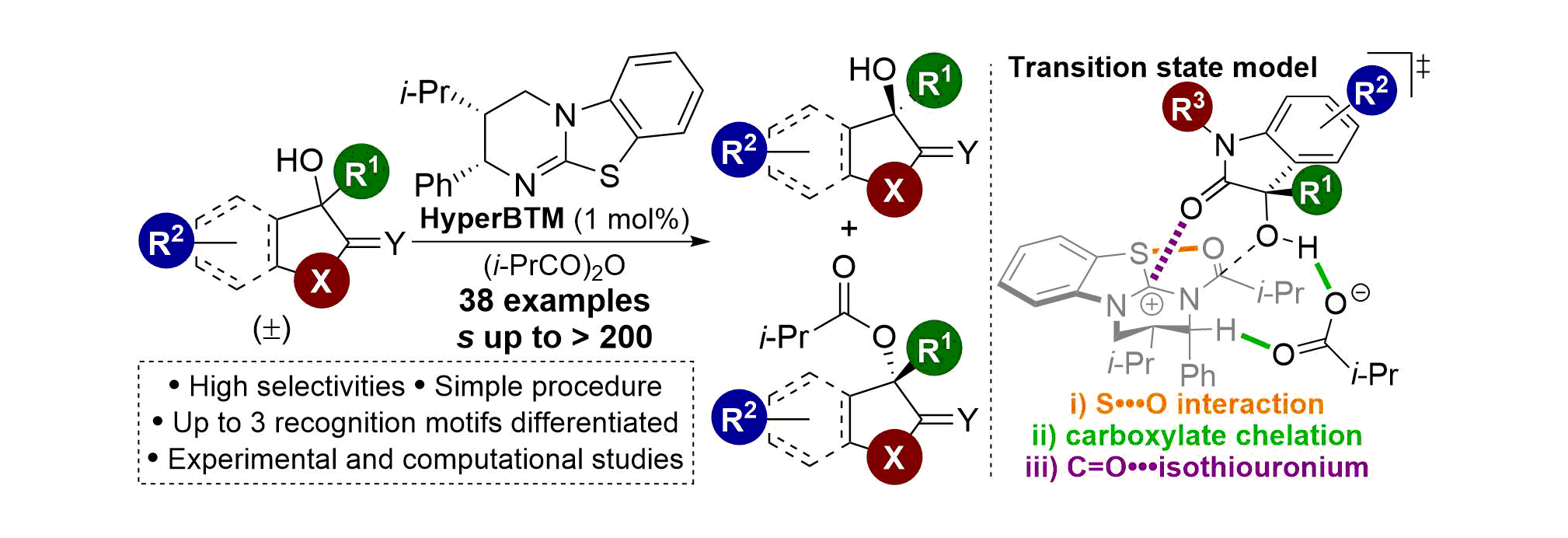
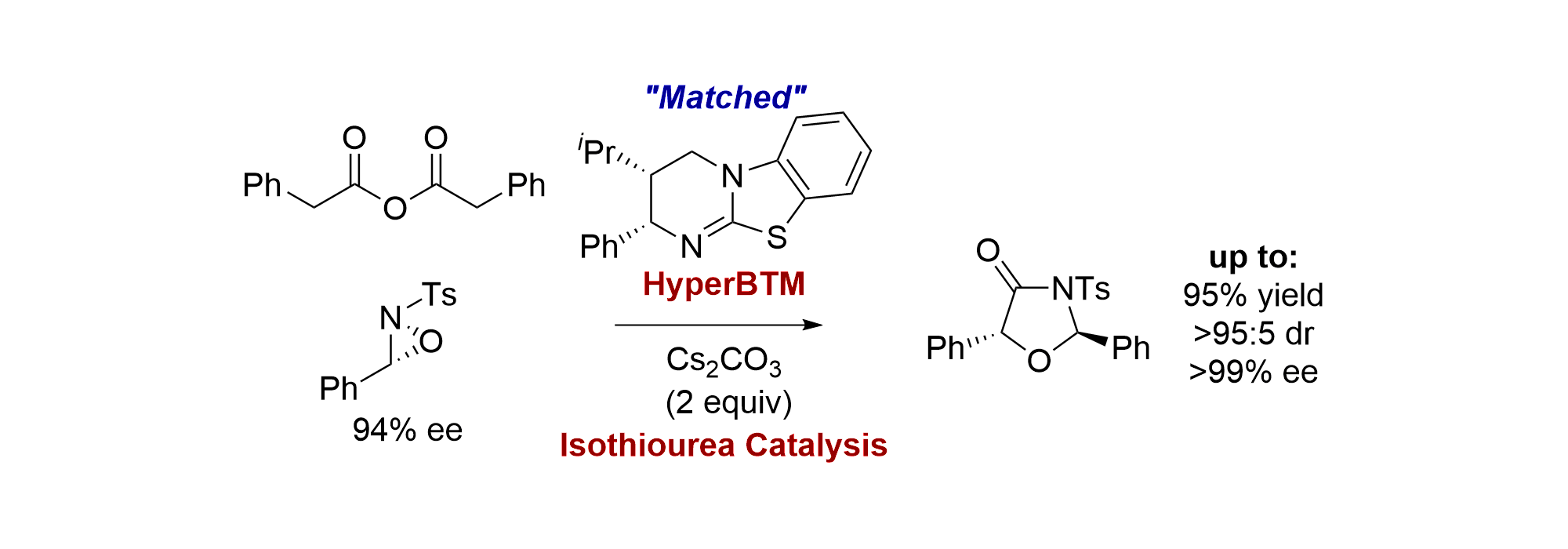
134. Scope, limitations and mechanistic analysis of the HyperBTM-catalyzed acylative kinetic resolution of tertiary heterocyclic alcohols
S. M. Smith, M. D. Greenhalgh, T. Feoktistova, D. M. Walden, J. E. Taylor, D. B. Cordes, A. M. Z. Slawin, P. H-Y. Cheong and A. D. Smith
133. Isothiourea-Catalyzed Enantioselective α-Alkylation of Esters via 1,6-Conjugate Addition to para-Quinone Methides
J. N. Arokianathar, W. C. Hartley, C. McLaughlin, M. D. Greenhalgh, D. Stead, S. Ng, A. M. Z. Slawin, A. D. Smith
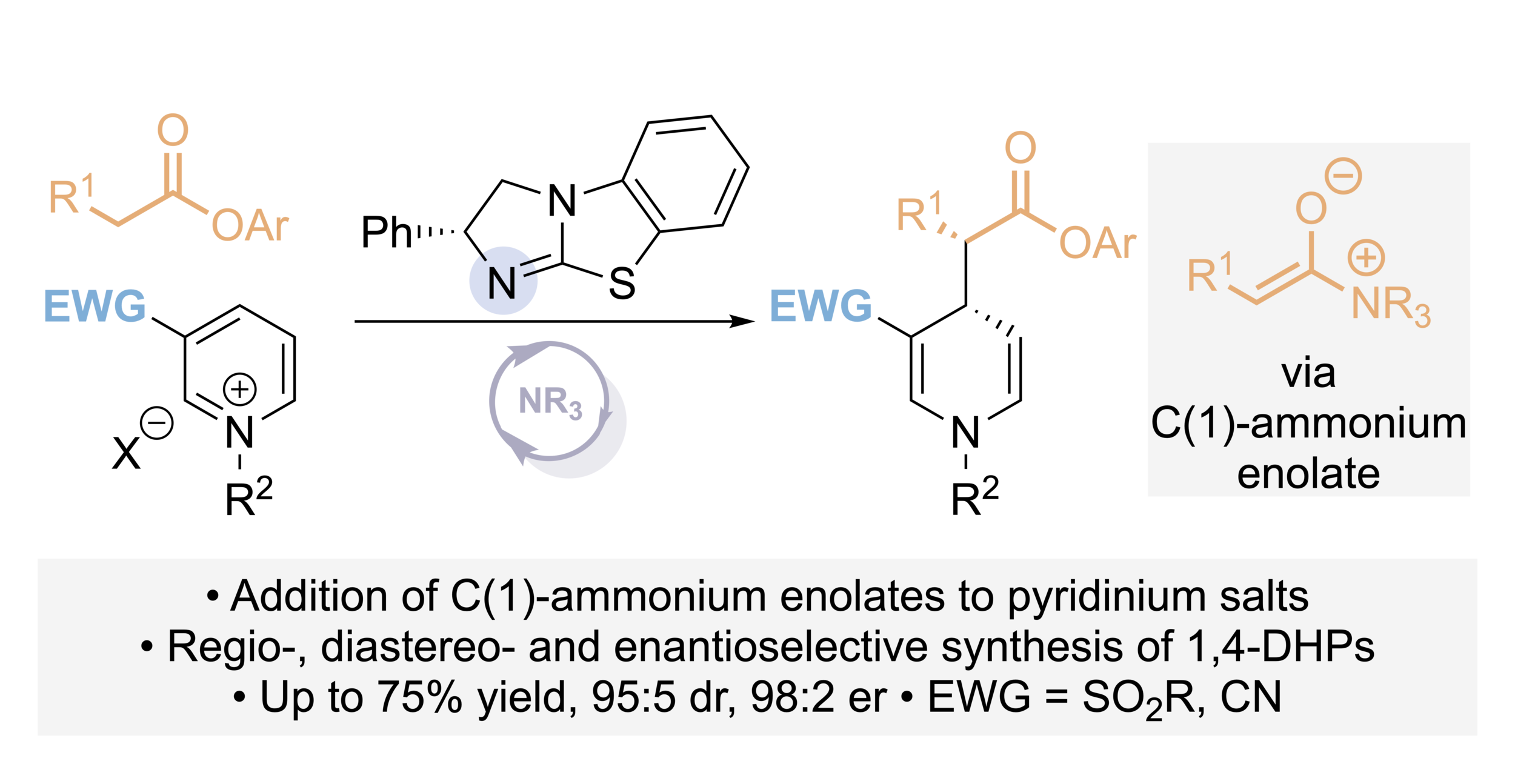
132. Catalytic enantioselective synthesis of 1,4- dihydropyridines via the addition of C(1)-ammonium enolates to pyridinium salts
C. McLaughlin, J. Bitai, L J. Barber, A. M. Z. Slawin and A. D. Smith
131. Kinetic and Structure-Activity Studies of the Triazolium Ion-catalyzed Stetter Reaction
C. J. Collett, C. M. Young, R. S. Massey, A. O'Donoghue, A. D. Smith
130. In vitro and in cellulo anti-diabetic activity of Au(I)-and Au(III)-isothiourea complexes
S. Fayyaz, M. Shaikh, D. Gasperini, S. P. Nolan, A. D. Smith, M. I. Choudhary
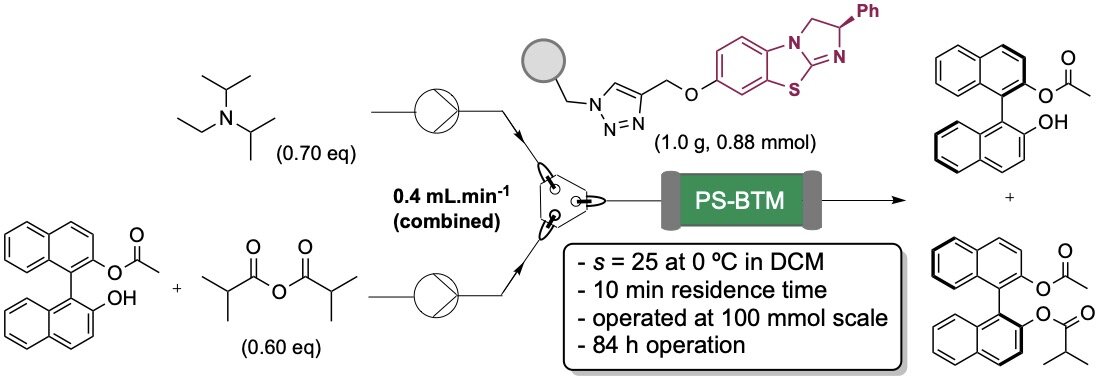
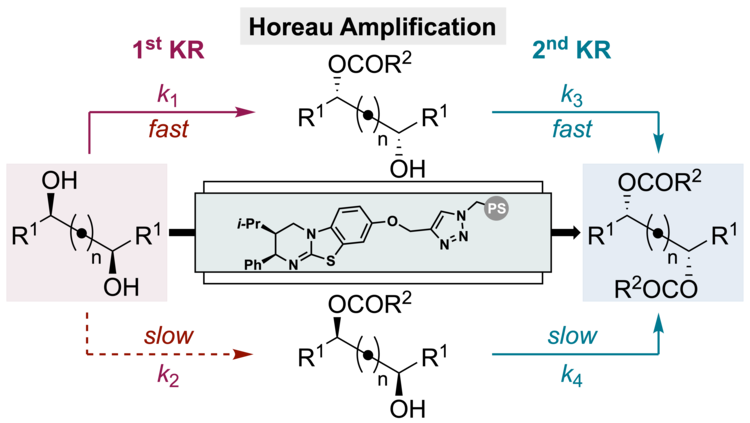
129. Horeau Amplification in the Sequential Acylative Kinetic Resolution of (±)-1,2-Diols and (±)-1,3-Diols in Flow
A. Brandolese, M. D. Greenhalgh, S. Qu, T. Desrues, X. Liu, C. Bressy, A. D. Smith
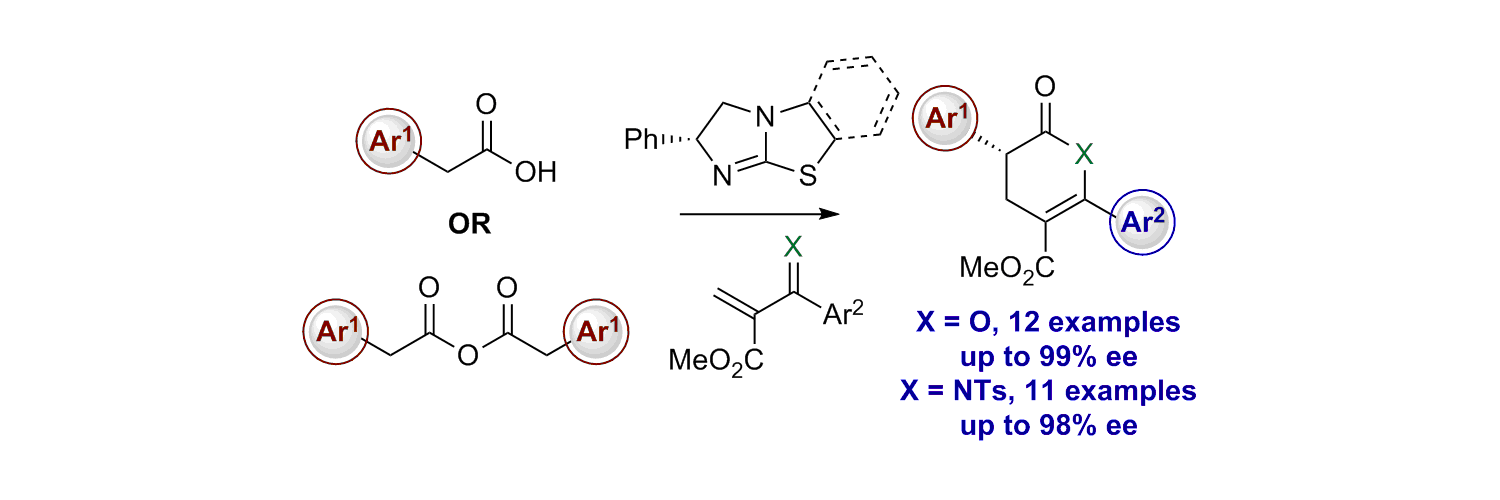
128. Enantioselective Synthesis of α-Aryl-β2-Amino-esters via Cooperative Isothiourea and Brønsted Acid Catalysis
F. Zhao; C. Shu; C. M. Young; C. Carpenter-Warren; A. M. Z. Slawin, A. D. Smith
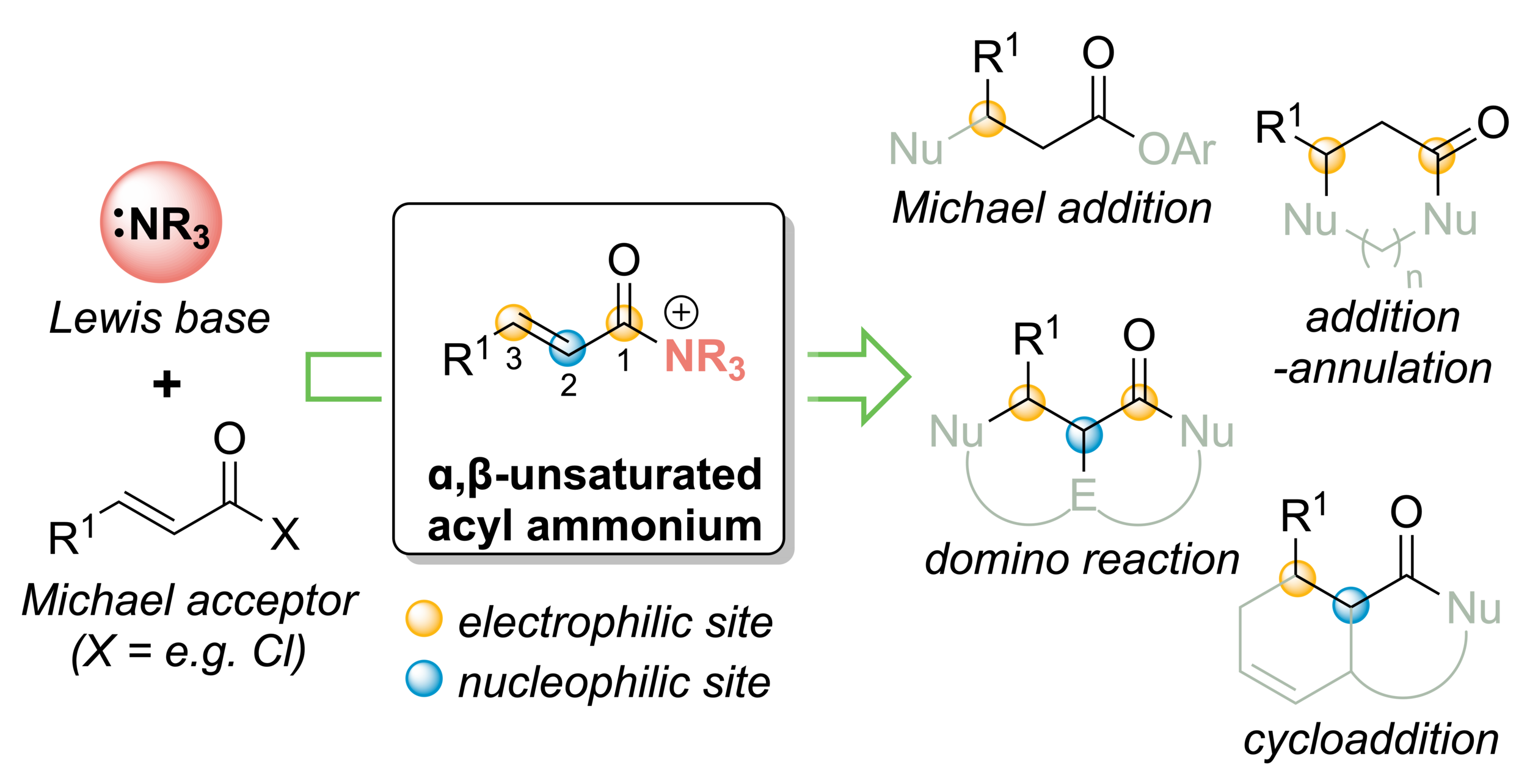
127. α,β-Unsaturated acyl ammonium species as reactive intermediates in organocatalysis: an update
J. Bitai, M. Westwood, A. D. Smith
126. Ultrarapid Cerium(III)–NHC Catalysts for High Molar Mass Cyclic Polylactide
P. M. D. A. Ewing, R. W. F. Kerr, S. K. Raman, A. D. Smith, C. K. Williams, and P. L. Arnold
125. Kinetic and Structure-Activity Studies of the Triazolium Ion-catalysed Benzoin Condensation
R. Massey, J. Murray, C. Collett, J. Zhu, A. D. Smith, A. O'Donoghue
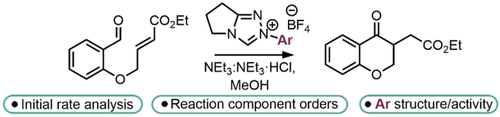
124.Kinetic resolution and desymmetrization of alcohols and amines by nonenzymatic, enantioselective acylation
A. B. Frost, M. D. Greenhalgh, E. S. Munday, S. F. Musolino, J. E. Taylor, A. D. Smith
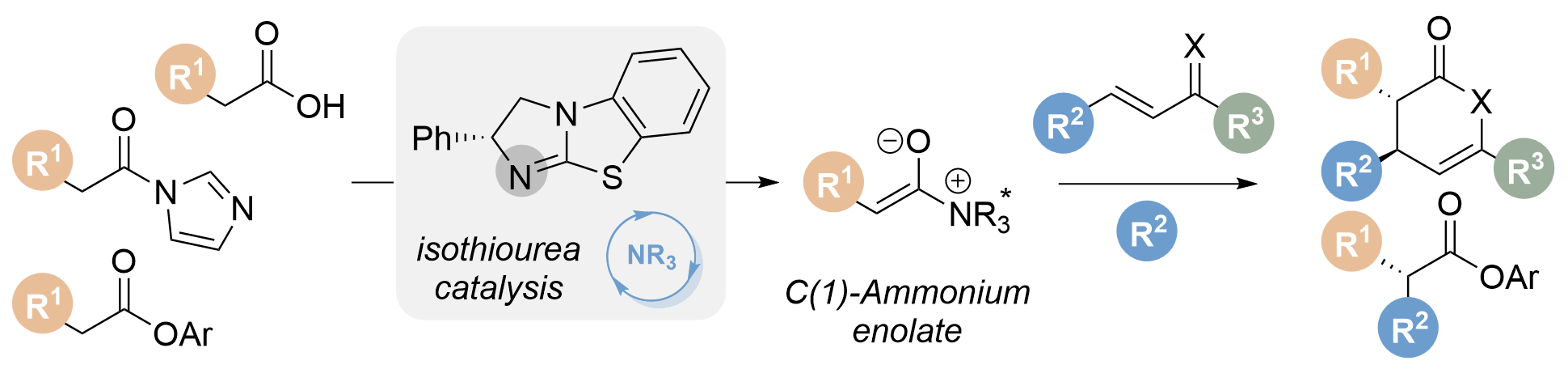
123. Generation and Reactivity of C(1)-Ammonium Enolates Using Isothiourea Catalysis
C. McLaughlin and A. D. Smith
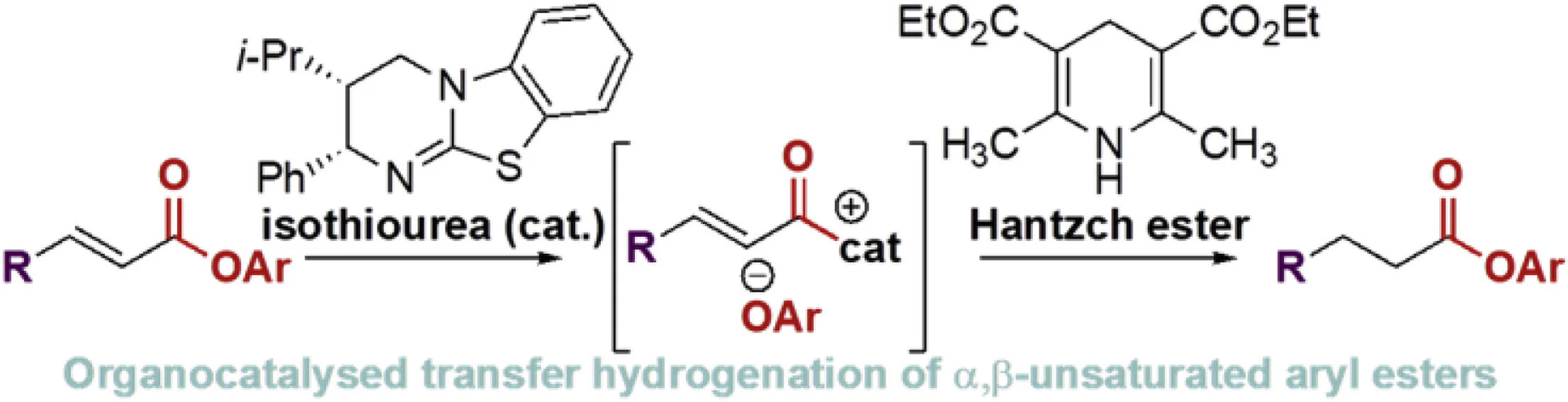
122. Isothiourea-catalyzed transfer hydrogenation of α,β-unsaturated para-nitrophenyl esters
J. Wu, C. M. Young, A. D. Smith
121. A retrospective cross-sectional study to determine chirality status of registered medicines in Tanzania
K. W. Mwamwitwa, R. M. Kaibere, A. M. Fimbo, W. Sabitii, N. E. Ntinginya, B. T. Mmbaga, D. H. Shewiyo, M. C. Shearer, A. D. Smith, E. A. Kaale
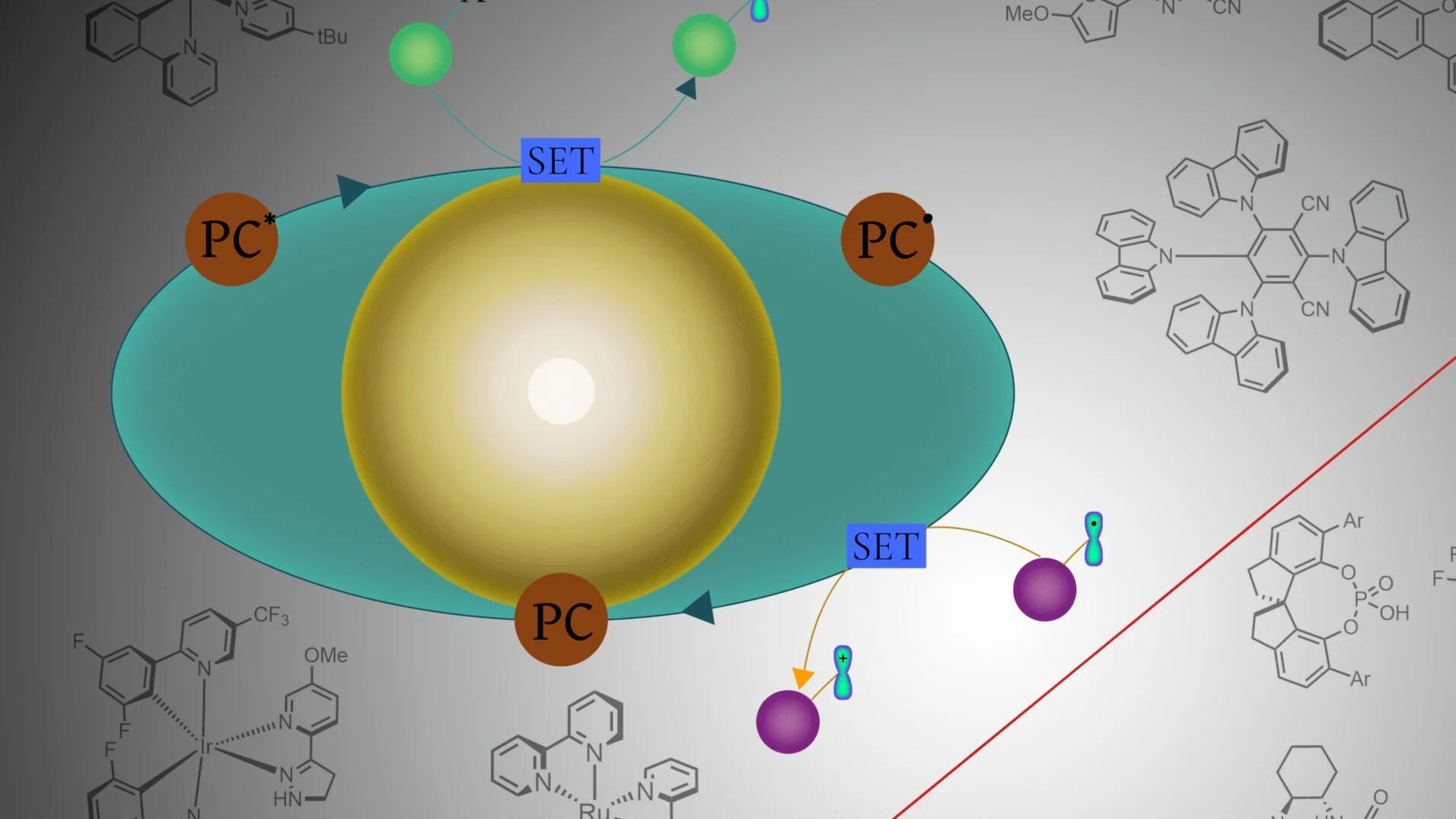
120. Recent developments in enantioselective photocatalysis
C. Prentice, J. Morrison, E. Zysman-Colman and A. D. Smith
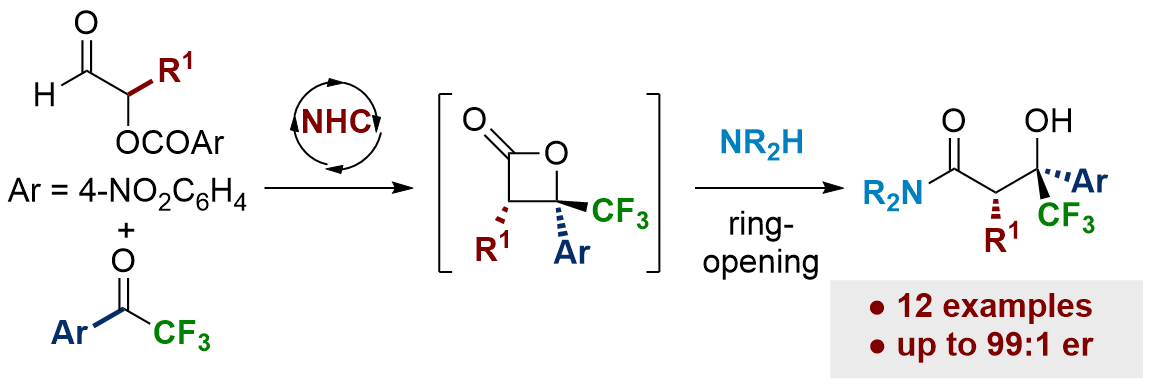
119. NHC-catalyzed enantioselective synthesis of β-trifluoromethyl-β-hydroxyamides
A. T. Davies, M. D. Greenhalgh, A. M. Z. Slawin and A. D. Smith
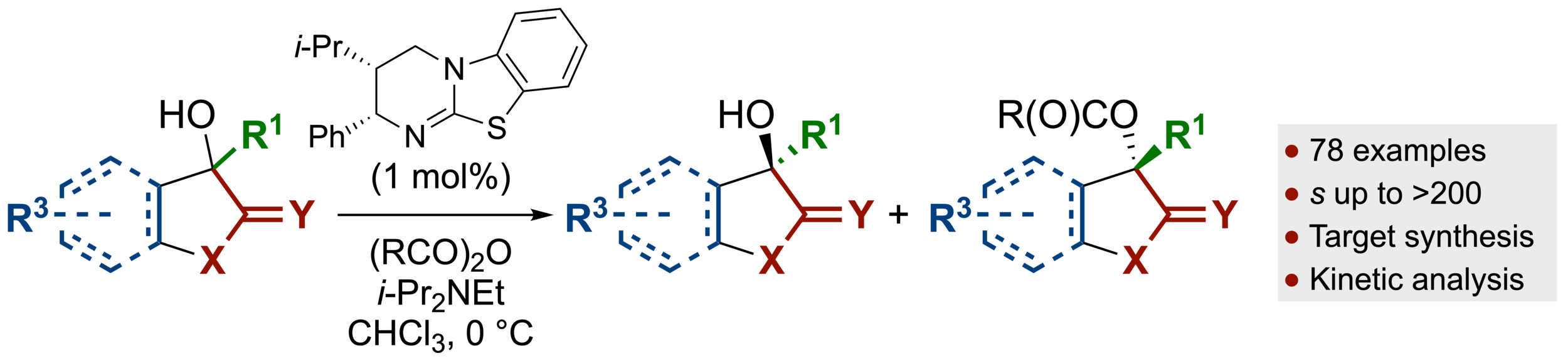
118. Isothiourea-Catalyzed Acylative Kinetic Resolution of Tertiary α-Hydroxy Esters
S. Qu, S.M. Smith, V. Laina-Martín, R. M. Neyyappadath, M. D. Greenhalgh and A. D. Smith
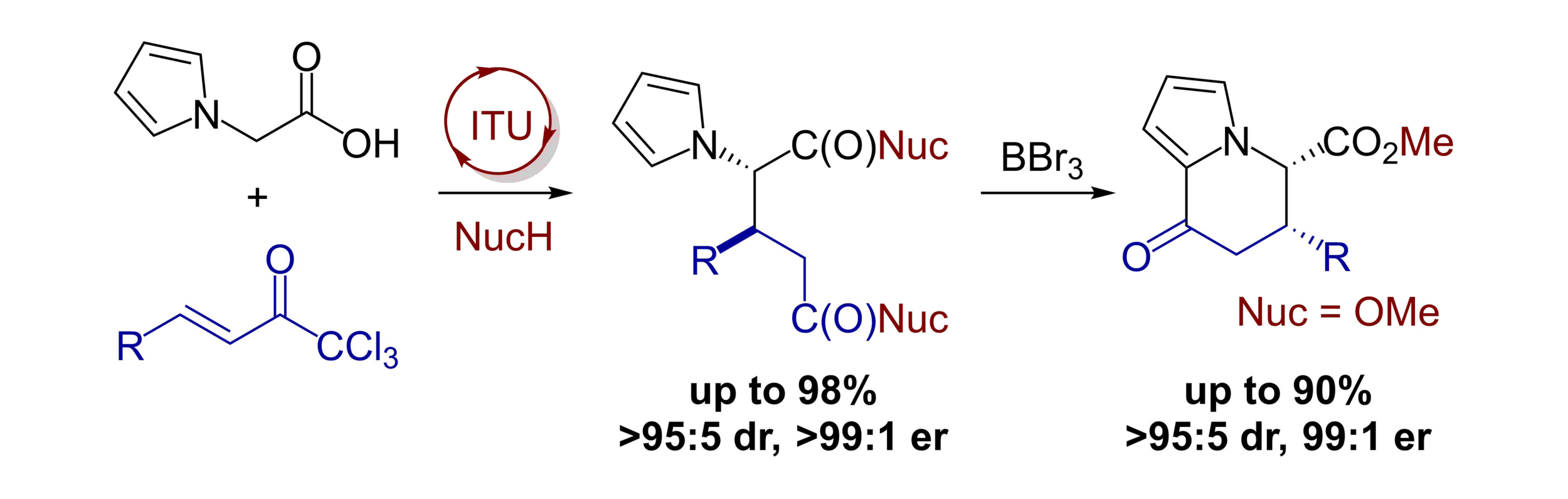
117. Isothiourea-catalyzed functionalization of pyrrolyl- and indolylacetic acid: enantioselective synthesis of dihydropyridinones and one-pot synthesis of pyridinones
S. Zhang, L. Bacheley, C. M. Young, D. G. Stark, T. O’Riordan, A. M. Z. Slawin and A. D. Smith
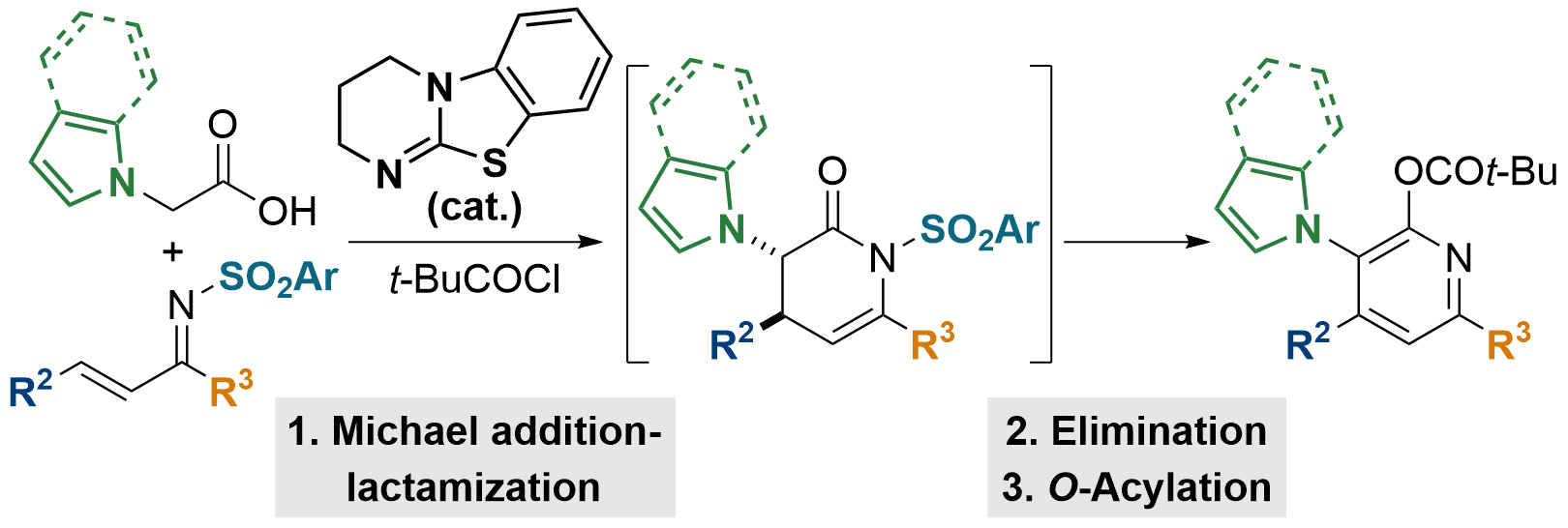
116. Isothiourea-Catalyzed Synthesis of Pyrrole- and Indole-Functionalized Tetrasubstituted Pyridines
S. Zhang, W. C. Hartley, M. D. Greenhalgh, S. Ng, A. M. Z. Slawin and A. D. Smith
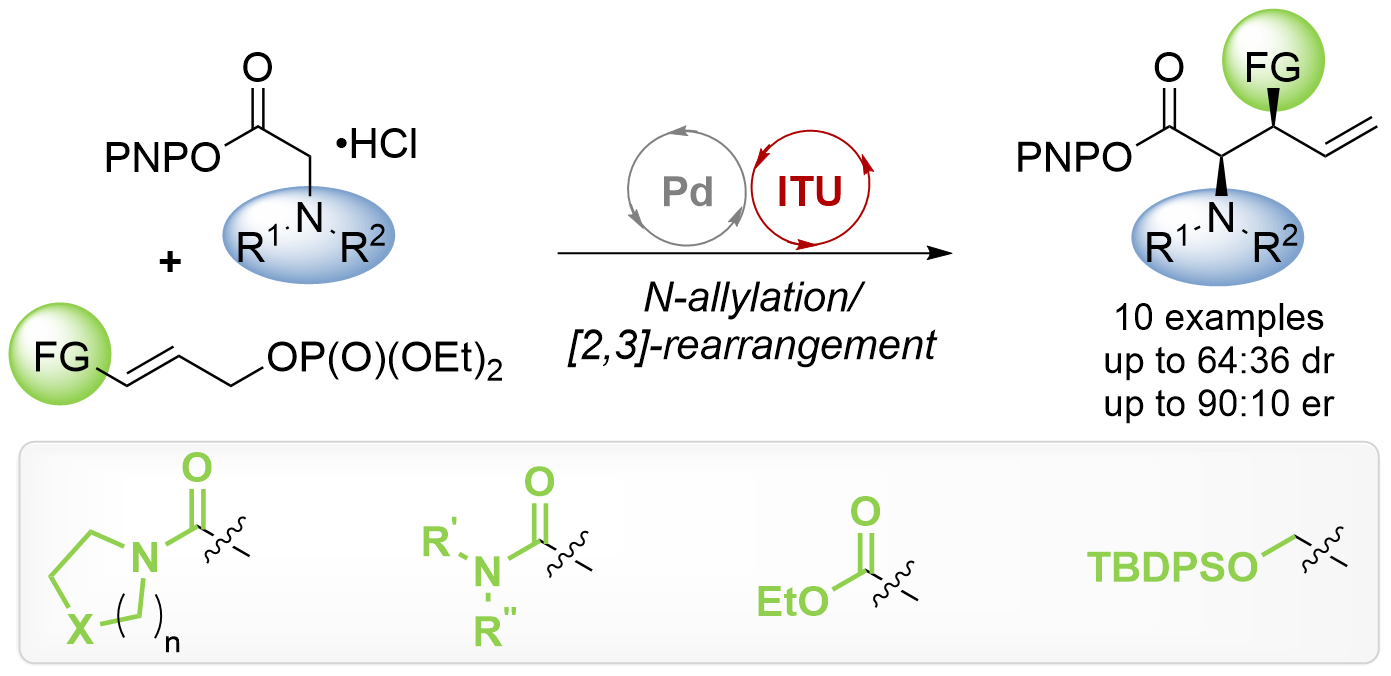
115. Exploring the Scope of Tandem Palladium and Isothiourea Relay Catalysis for the Synthesis of α-Amino Acid Derivatives
J. Bitai, A. M. Z. Slawin, D. B. Cordes, A. D. Smith
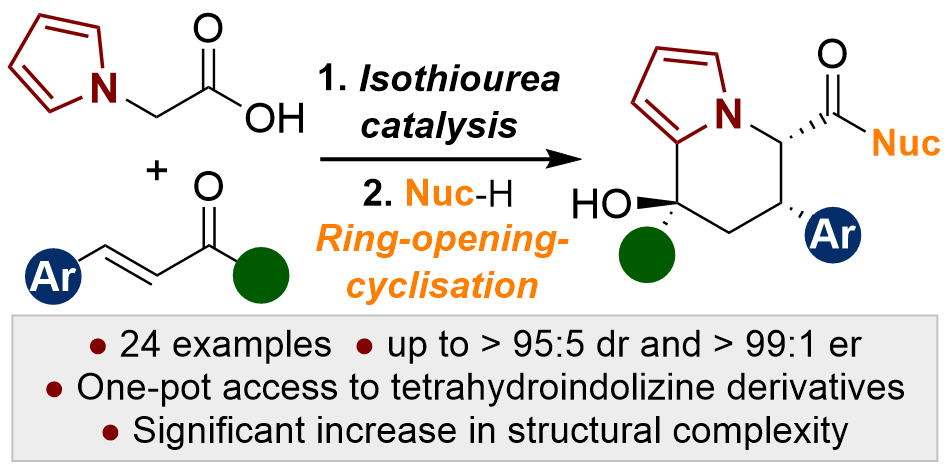
114. Tandem sequential catalytic enantioselective synthesis of highly-functionalised tetrahydroindolizine derivatives
S. Zhang, M. D. Greenhalgh, A. M. Z. Slawin, A. D. Smith
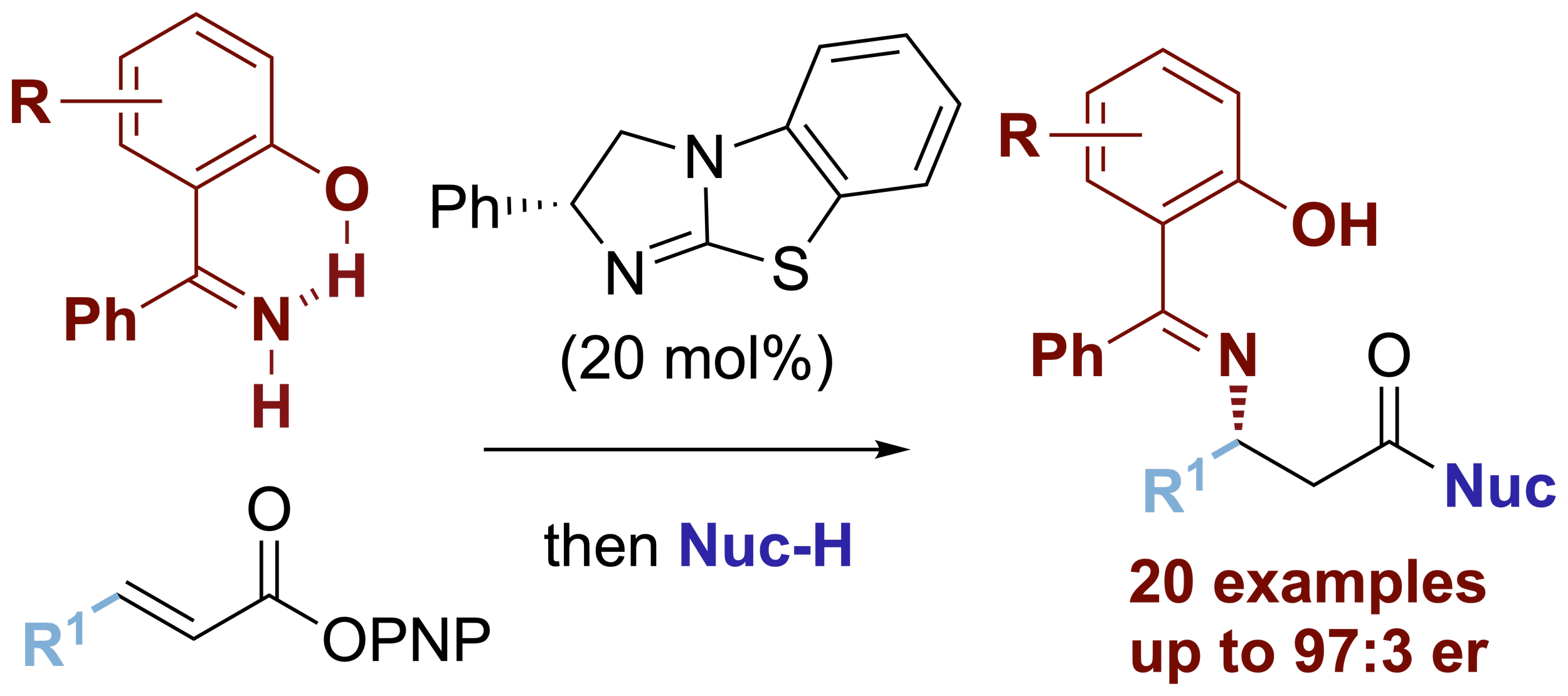
113. Isothiourea-Catalyzed Atropselective Acylation of Biaryl Phenols via Sequential Desymmetrization / Kinetic Resolution
E. S. Munday, M. A. Grove, T. Feoktistova, A. C. Brueckner, D. M. Walden, C. M. Young, A. M. Z. Slawin, A. D. Campbell, P. H.-Y. Cheong and A. D. Smith
112. Isothiourea-Catalyzed Enantioselective Synthesis of Tetrahydro-𝛂-carbolinones
H. Liu, A. M. Z. Slawin and A. D. Smith
111. A Mechanistically and Operationally Simple Route to Metal-N-Heterocyclic Carbene (NHC) Complexes
N. V. Tzouras, F. Nahra, L. Falivene, L. Cavallo, M. Saab, K. Van Hecke, A. Collado, C. J. Collett, A. D. Smith, C. S. J. Cazin and S. P. Nolan
110. Continuous Flow Preparation of Enantiomerically Pure BINOL(s) by Acylative Kinetic Resolution
J. Lai, R. M. Neyyappadath, A. D. Smith and M. A. Pericàs
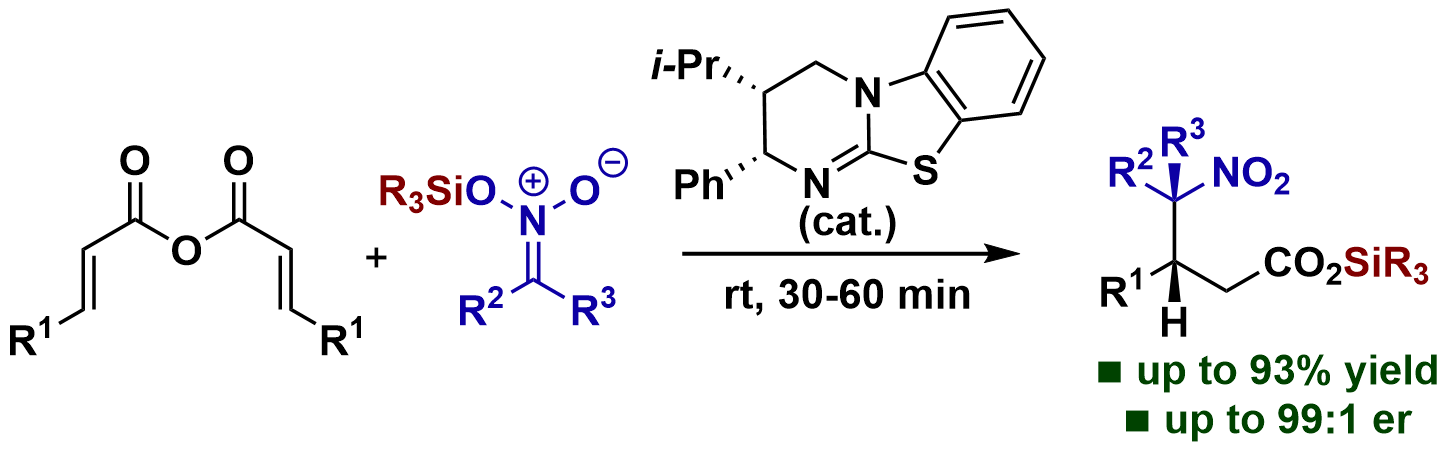
109. Unanticipated Silyl Transfer in Enantioselective α,β-Unsaturated Acyl Ammonium Catalysis Using Silyl Nitronates
A. Matviitsuk, M. D. Greenhalgh, J. E. Taylor, X. B. Nguyen, D. B. Cordes, A. M. Z. Slawin, D. W. Lupton, A. D. Smith
108. The Importance of 1,5-Oxygen•••Chalcogen Interactions in Enantioselective Isochalcogenourea Catalysis
C. M. Young, A. Elmi, D. J. Pascoe, R. K. Morris, C. McLaughlin, A. M. Woods, A. B. Frost, A. de la Houpliere, K. B. Ling, T. K. Smith, A. M. Z. Slawin, P. H. Willoughby, S. L. Cockroft and A. D. Smith
107. NHC-Catalysed Enantioselective Intramolecular Formal [4+2] Cycloadditions using Carboxylic Acids as Azolium Enolate Precursors
N. Attaba and A. D. Smith
106. Isothiourea-catalysed enantioselective addition of N-heterocycles to α,β-unsaturated aryl esters
C. Shu, H. Liu, A. M. Z. Slawin, C. Carpenter-Warren and A. D. Smith
105. Base-free Enantioselective C(1)-Ammonium Enolate Catalysis Exploiting Aryloxides: A Synthetic and Mechanistic Study
C. McLaughlin, A. M. Z. Slawin and A. D. Smith
104. Isothiourea-Catalysed Sequential Kinetic Resolution of Acyclic (±)-1,2-Diols
S. Harrer, M. D. Greenhalgh, R. M. Neyyappadath and A. D. Smith
103. Catalytic enantioselective synthesis of perfluoroalkyl-substituted β-lactones via a concerted asynchronous [2+2] cycloaddition: A synthetic and computational study
D. Barrios Antunez, M. D. Greenhalgh, A. C. Brueckner, D. M. Walden, P. Elías-Rodríguez, P. Roberts, B. Young, T. W. West, A. M. Z. Slawin, P. H-Y. Cheong and A. D. Smith
102. Evaluating aryl esters as bench-stable C(1)-ammonium enolate precursors in catalytic, enantioselective Michael addition–lactonisations
C. M. Young, J. E. Taylor and A. D. Smith
101. Synthesis of Fused Indoline-Cyclobutanone Derivatives via an Intramolecular [2+2] Cycloaddition
R. M. Neyyappadath, M. D. Greenhalgh, D. B. Cordes, A. M. Z. Slawin and A. D. Smith
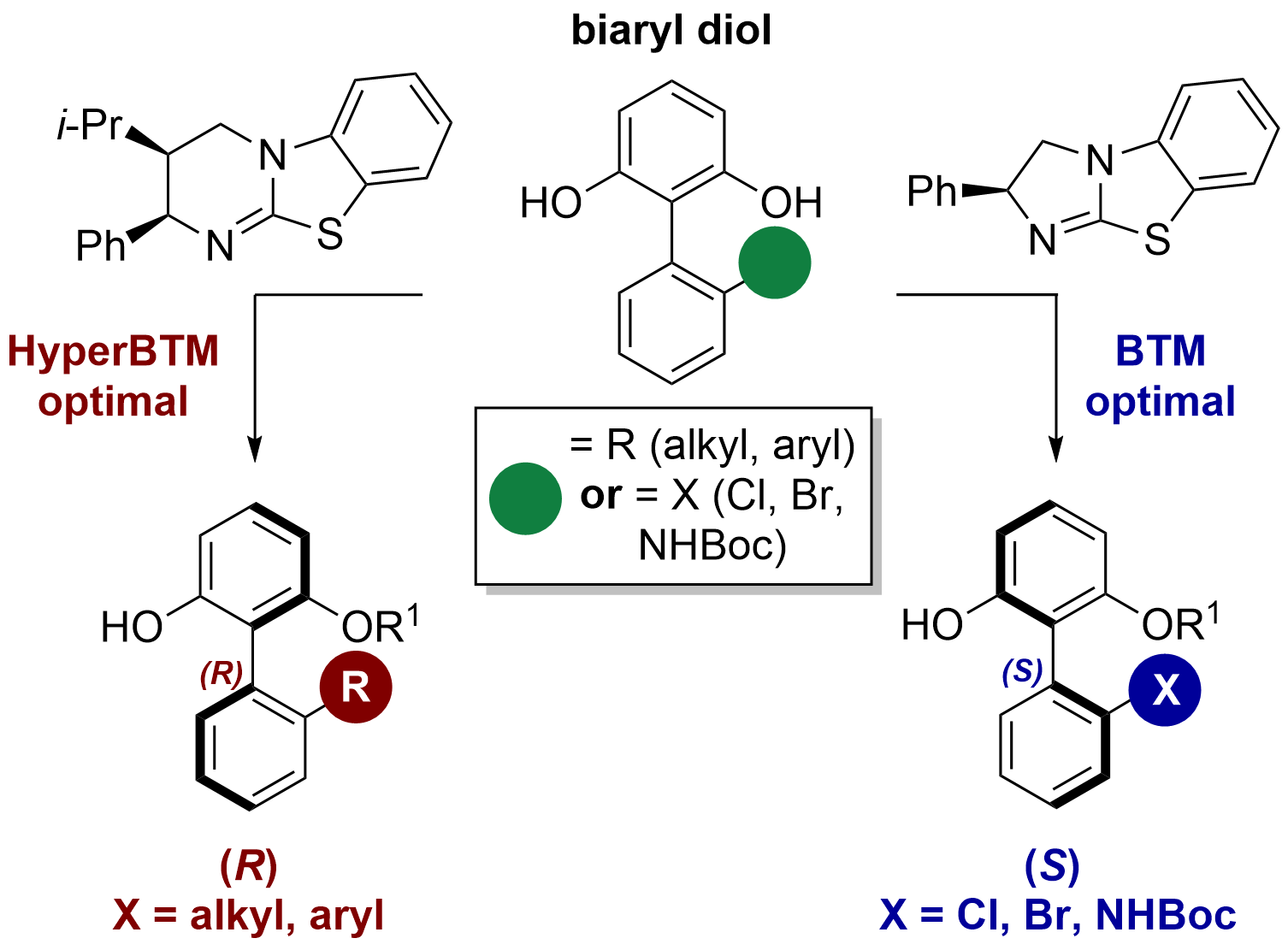
100. Isothiourea-Catalyzed Regioselective Acylative Kinetic Resolution of Axially Chiral Biaryl Diols
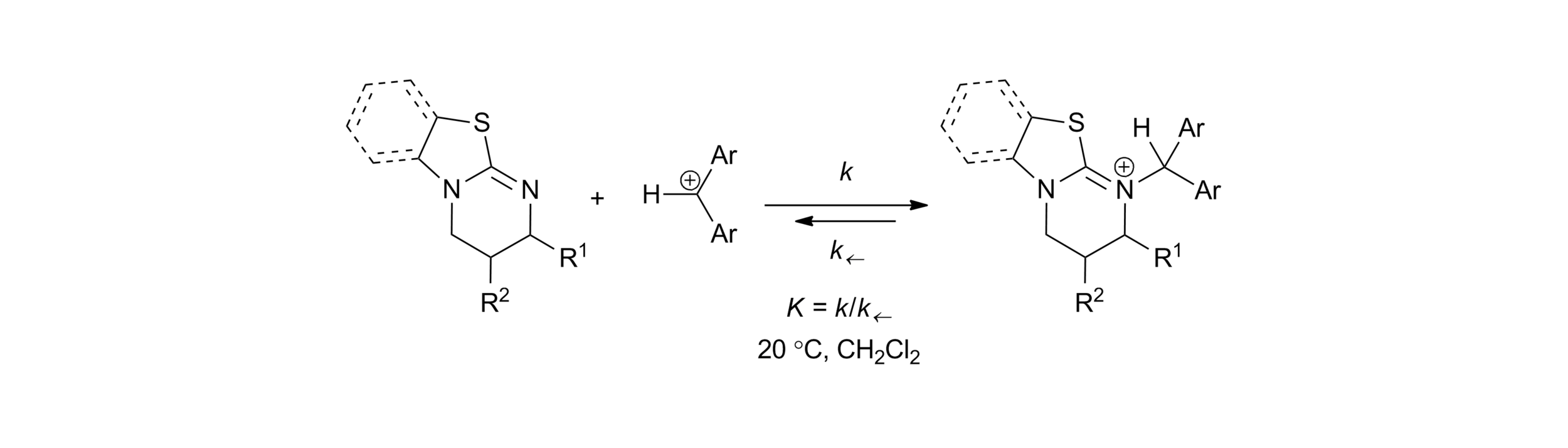
S. Qu, M. D. Greenhalgh and A. D. Smith
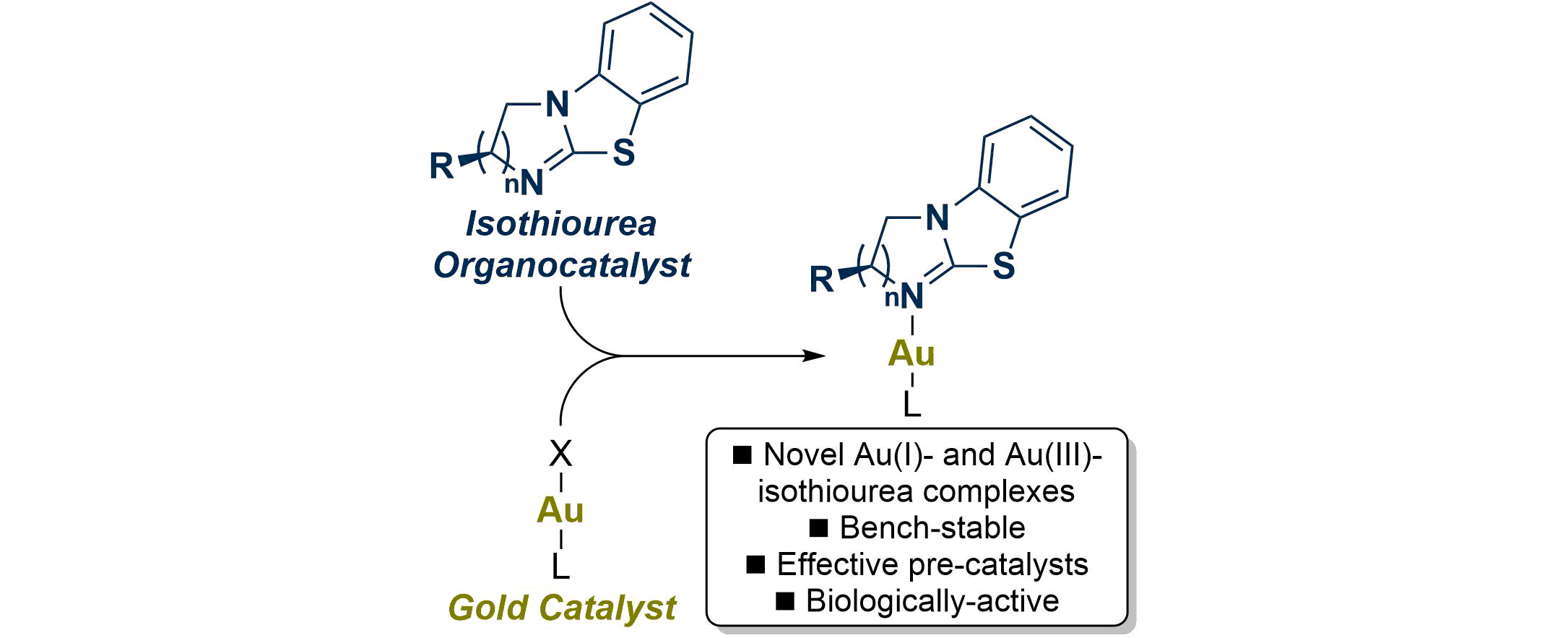
99. Chiral Au(I) and Au(III)-Isothiourea Complexes: Synthesis, Characterization and Application
D. Gasperini, M. D. Greenhalgh, R Imad, S. Siddiqui, A. Malik, F. Arshad, M. I. Choudhary, A. Al-Majid, D. Cordes, A. M. Z. Slawin, S. Nolan and A. D. Smith
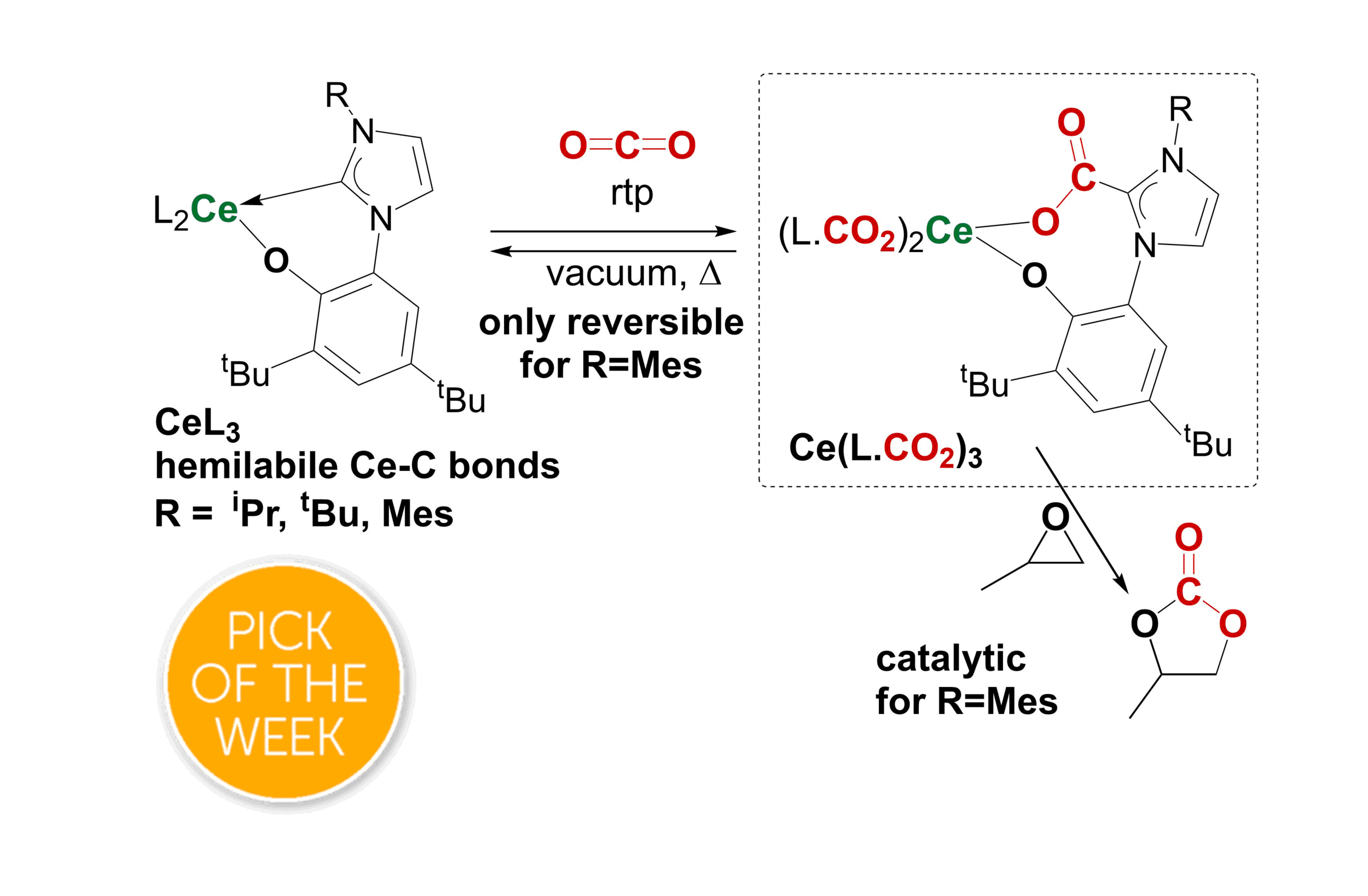
98. Selective and catalytic carbon dioxide and heteroallene activation mediated by cerium N-heterocyclic carbene complexes
P. L. Arnold, R. W. F. Kerr, C. Weetman, S. R. Docherty, J. Rieb, F. L. Cruickshank, K. Wang, C. Jandl, M. W. McMullon, A. Pöthig, F. E. Kühn and A. D. Smith
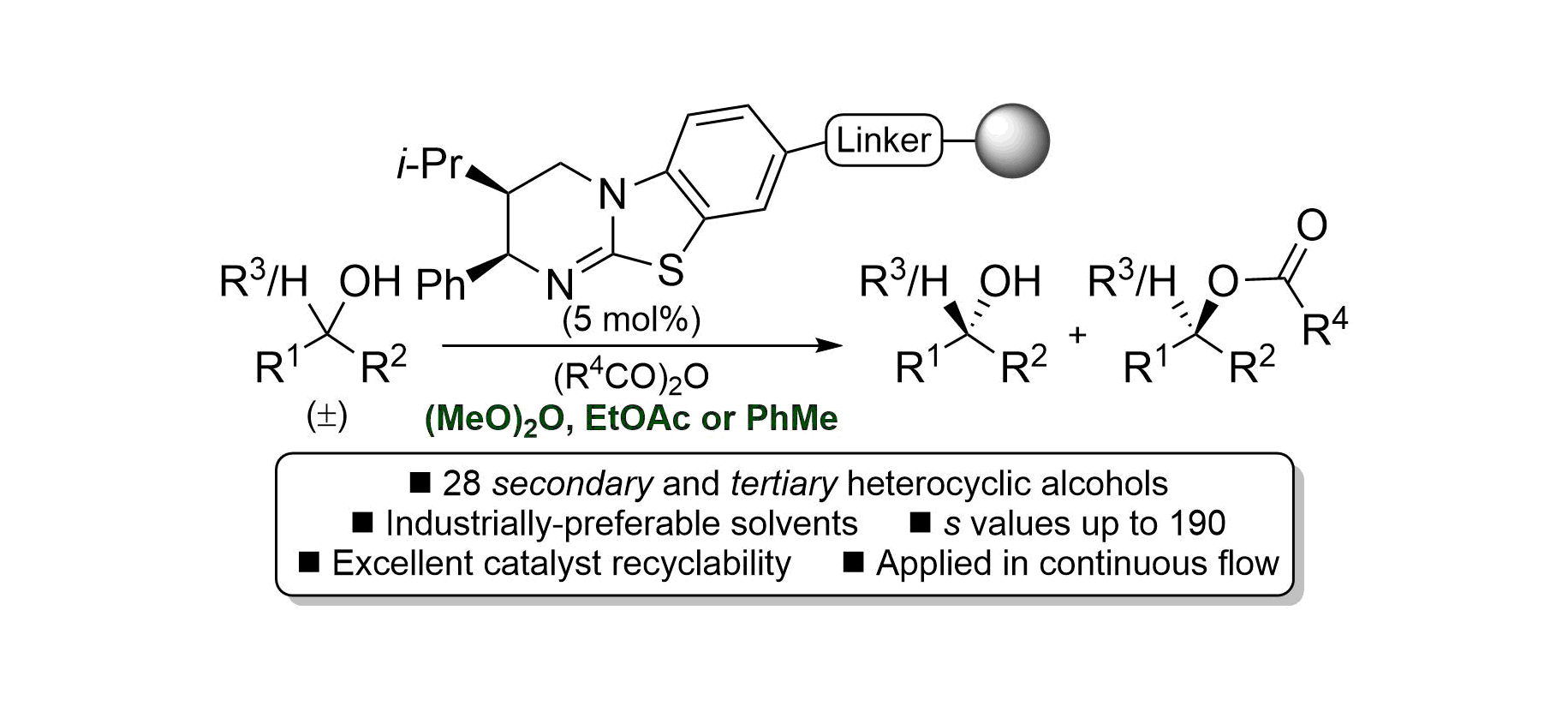
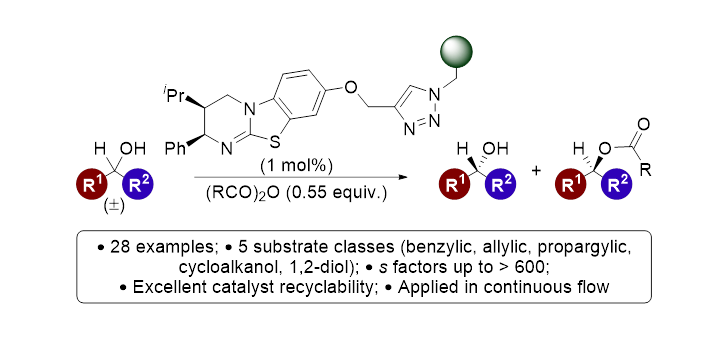
97. Evaluating polymer-supported isothiourea catalysis in industrially-preferable solvents for the acylative kinetic resolution of secondary and tertiary heterocyclic alcohols in batch and flow
N. R. Guha, R. M. Neyyappadath, M. D. Greenhalgh, R. Chisholm, S. M. Smith, M. L. McEvoy, C. M. Young, C. Rodríguez-Escrich, M. A. Pericàs, G. Hähner and A. D. Smith
96. Isothiourea-Catalyzed Enantioselective Functionalization of 2-Pyrrolyl Acetic Acid: Two-Step Synthesis of Stereodefined Dihydroindolizinones
S. Zhang, J. E. Taylor, A. M. Z. Slawin and A. D. Smith
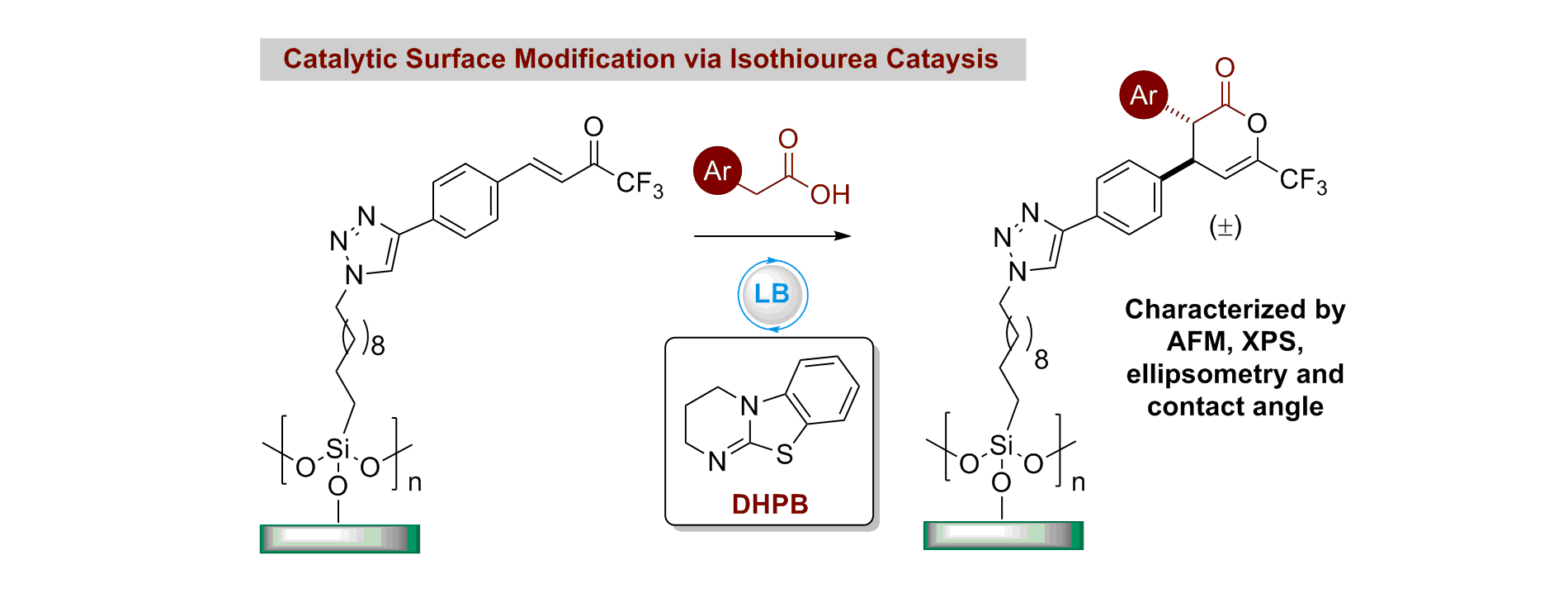
95. Direct organocatalytic enantioselective functionalization of SiOx surfaces
J. D. Parkin, R. Chisholm, A. B. Frost, R. G. Bailey, A. D. Smith and G. Hähner
94. Best practice considerations for using the selectivity factor, s, as a metric for the efficiency of kinetic resolutions
M. D. Greenhalgh, J. E. Taylor and A. D. Smith
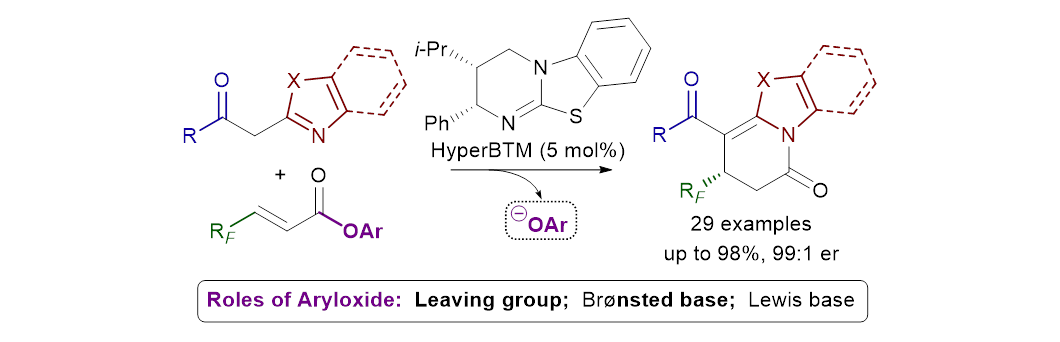
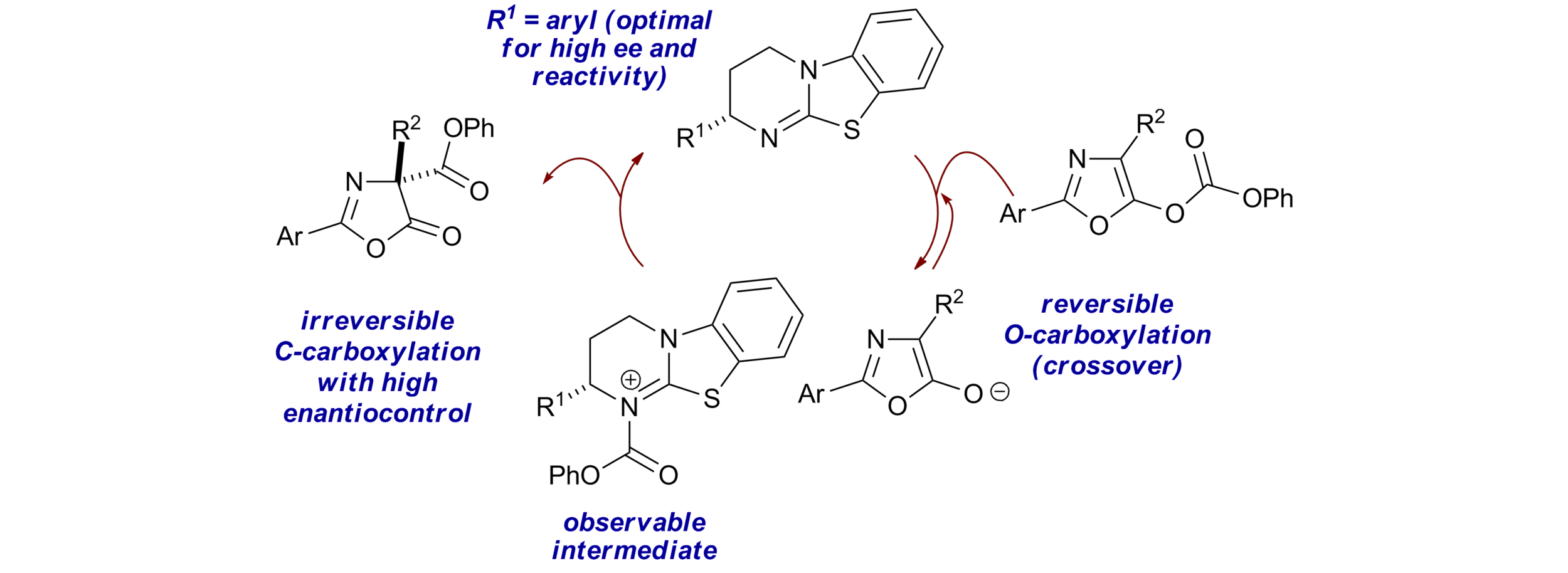
93. Multiple roles of aryloxide leaving groups in enantioselective annulations employing α,β-unsaturated acyl ammonium catalysis
M. D. Greenhalgh, S. Qu, A. M. Z. Slawin and A. D. Smith
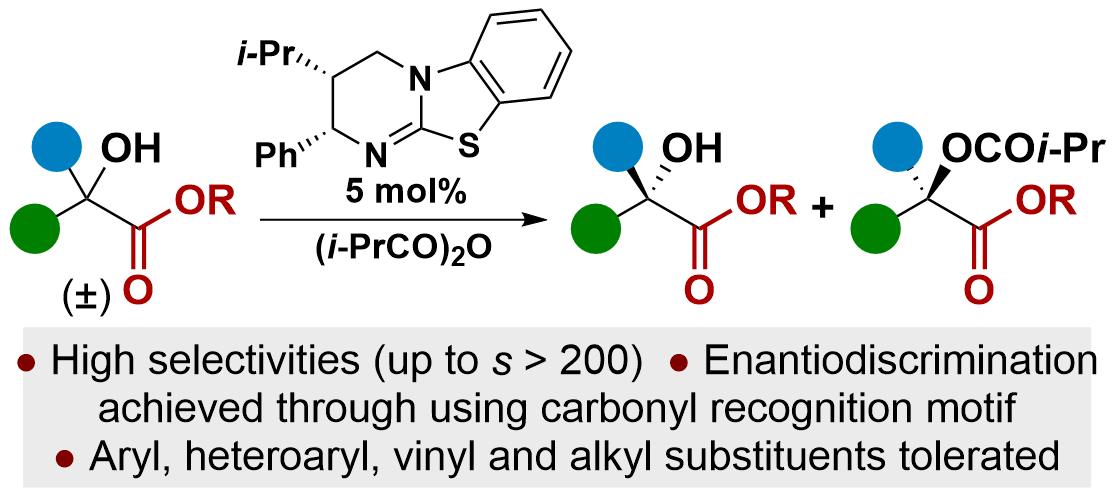
92. C=O•••Isothiouronium Interaction Dictates Enantiodiscrimination in Acylative Kinetic Resolution of Tertiary Heterocyclic Alcohols
M. D. Greenhalgh, S. M. Smith, D. M. Walden, J. E. Taylor, Z. Brice, E. R. T. Robinson, C. Fallan, D. B. Cordes, A. M. Z. Slawin, H. C. Richardson, M. A. Grove, P. H-Y. Cheong and A. D. Smith
91. Isothiourea-Catalyzed Enantioselective Addition of 4-Nitrophenyl Esters to Iminium Ions
J. N. Arokianathar, A. B. Frost, A. M. Z. Slawin, D. Stead and A. D. Smith
90. Acylative Kinetic Resolution of Alcohols Using a Recyclable Polymer-Supported Isothiourea Catalyst in Batch and Flow
R. M. Neyyappadath, R. Chisholm, M. D. Greenhalgh, C. Rodríguez-Escrich, M. A. Pericàs, G. Hähner and A. D. Smith
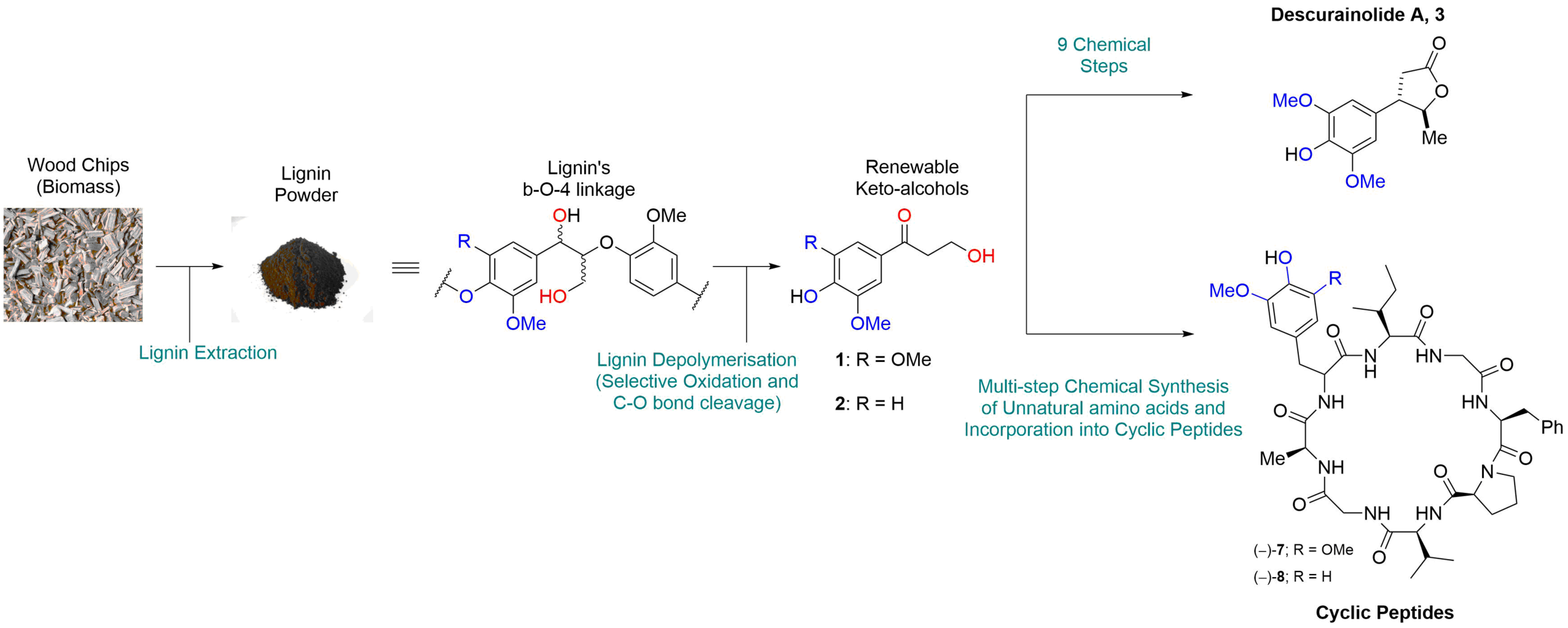
89. Synthesis of the natural product descurainolide and cyclic peptides from lignin-derived aromatics
O. S. Ojo, B. Nardone, S. F. Musolino, A. R. Neal, L. Wilson, T. Lebl, A. M. Z. Slawin, D. B. Cordes, J. E. Taylor, J. H. Naismith, A. D. Smith and N. J. Westwood
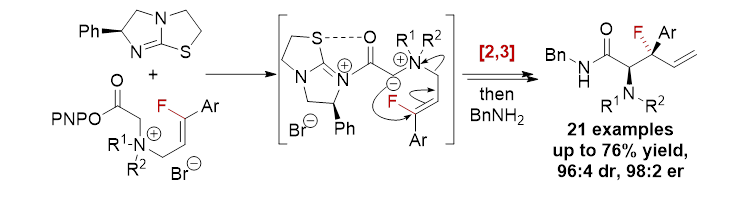
88. Enantioselective Synthesis of β-Fluoro-β-aryl-α-aminopentenamides by Organocatalytic [2,3]-Sigmatropic Rearrangement
K. Kasten, A. M. Z. Slawin and A. D. Smith
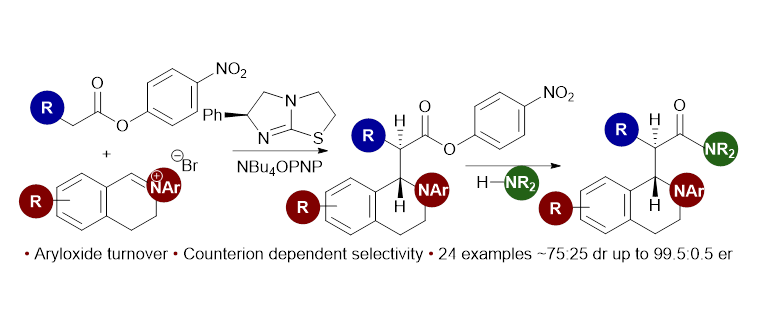
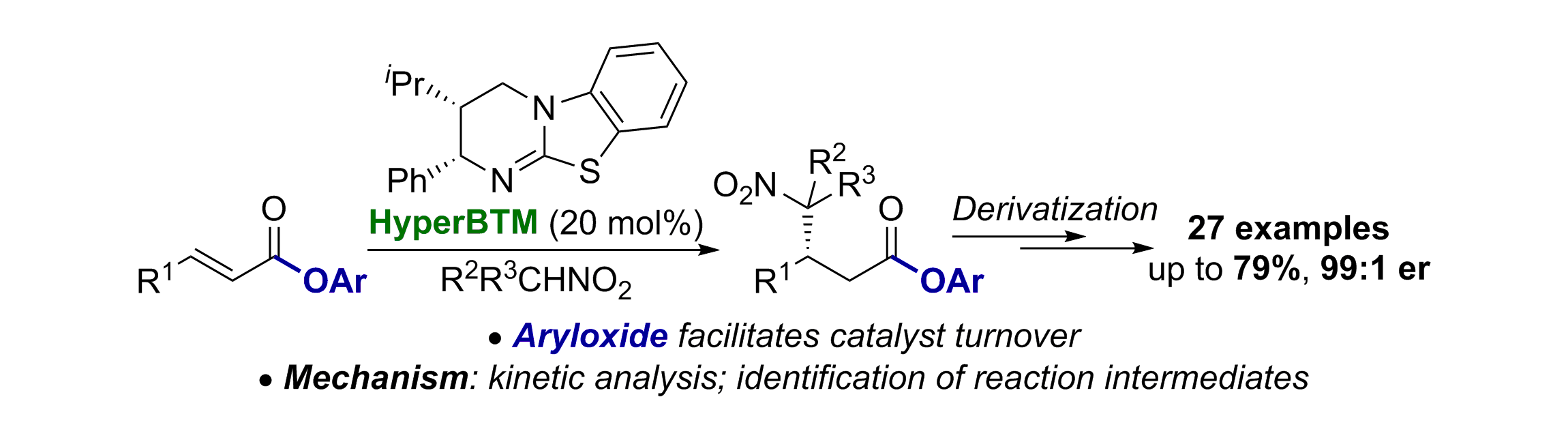
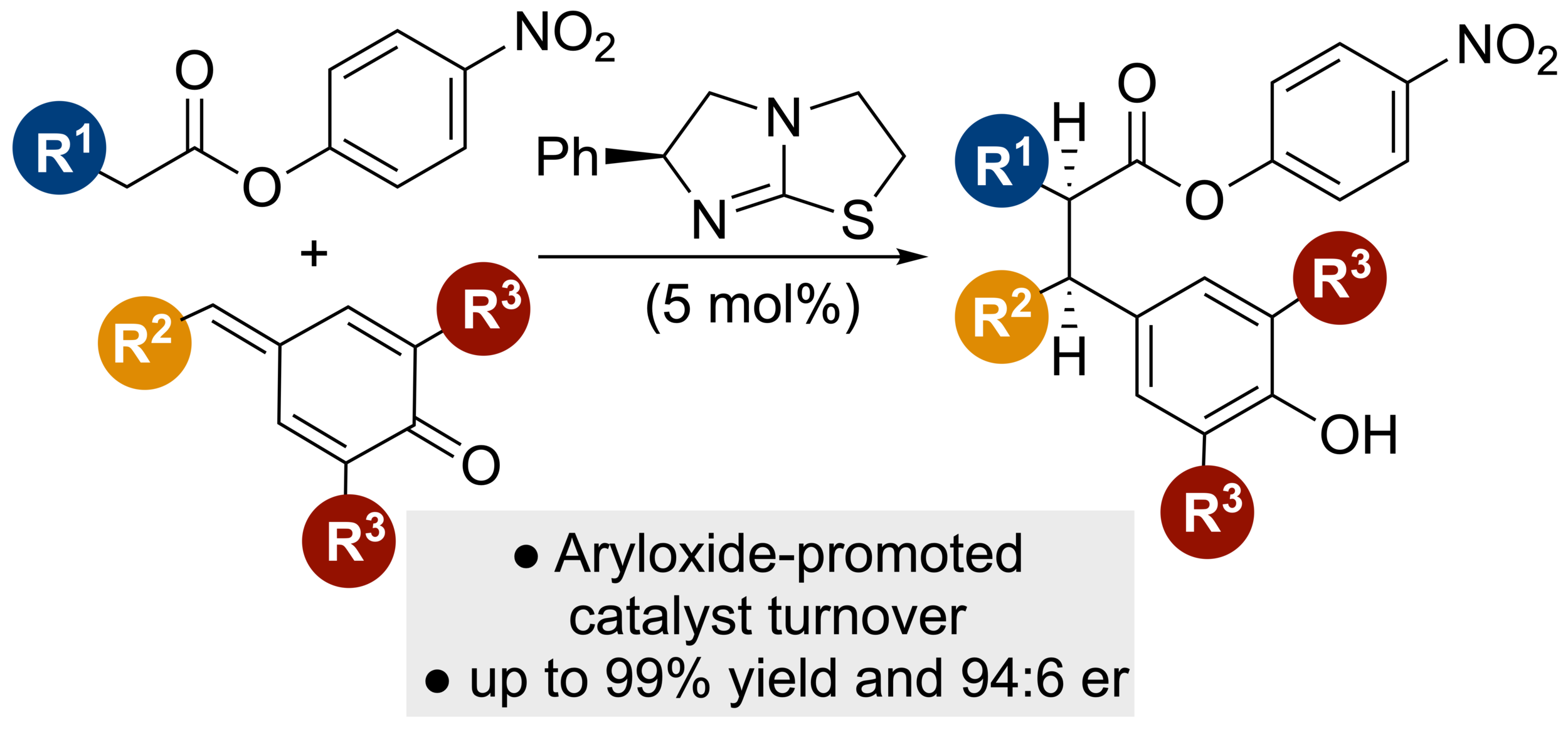
87. Aryloxide-Facilitated Catalyst Turnover in Enantioselective α,β-Unsaturated Acyl Ammonium Catalysis
A. Matviitsuk, M. D. Greenhalgh, D.-J. Barrios Antúnez, A. M. Z. Slawin and A. D. Smith
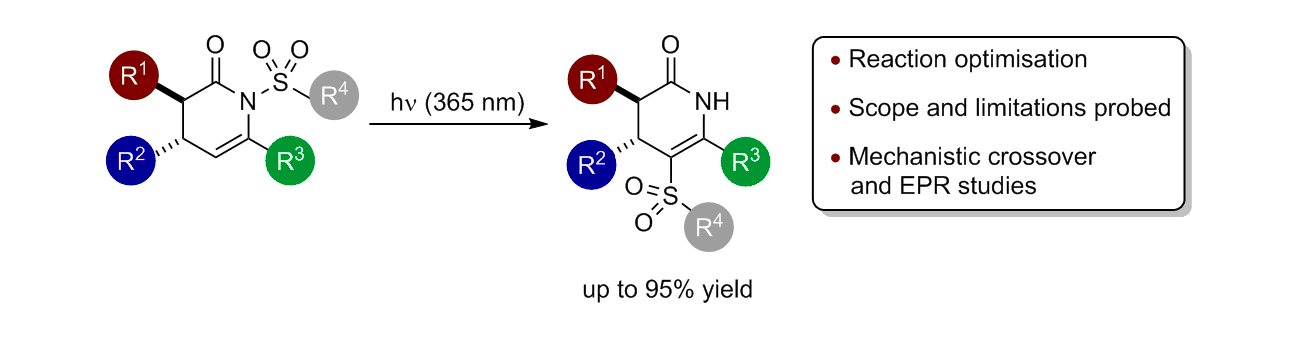
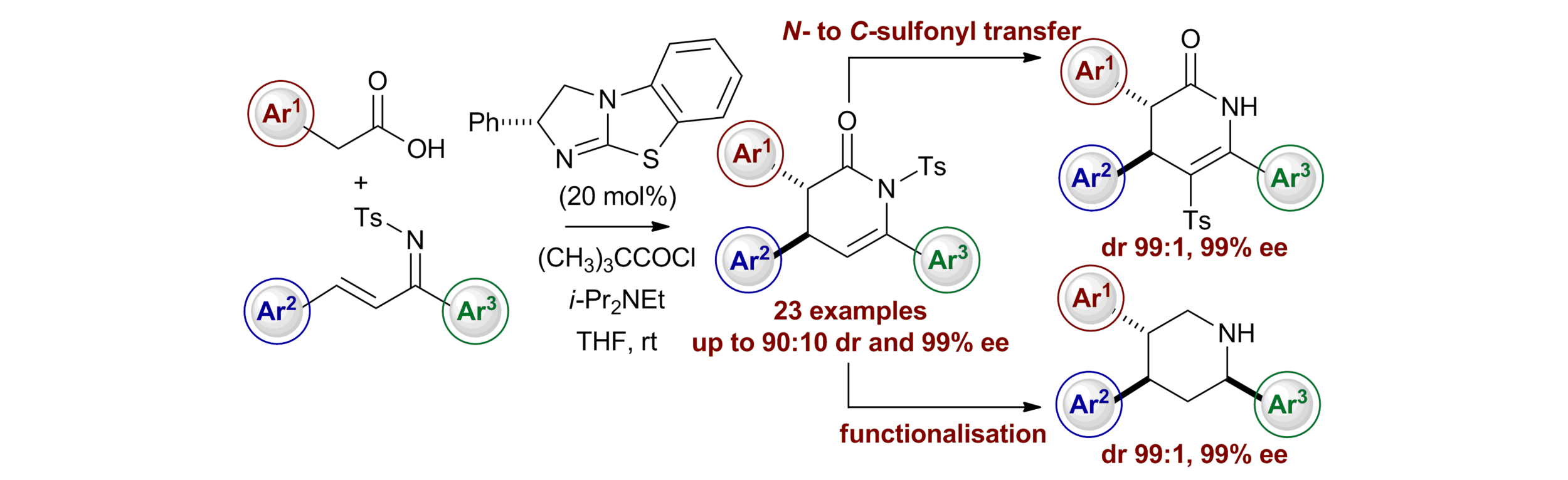
86. N- to C-Sulfonyl Photoisomerisation of Dihydropyridinones: A Synthetic and Mechanistic Study
P.-P. Yeh, J. E. Taylor, D. G. Stark, D. S. B. Daniels, C. Fallan, J. C. Walton and A. D. Smith
85. Tandem Pd and Isothiourea Relay Catalysis: Enantioselective Synthesis of α-Amino Acid Derivatives via Allylic Amination and [2,3]-Sigmatropic Rearrangement
S. S. M. Spoehrle, T. H. West, J. E. Taylor, A. M. Z. Slawin, A. D. Smith
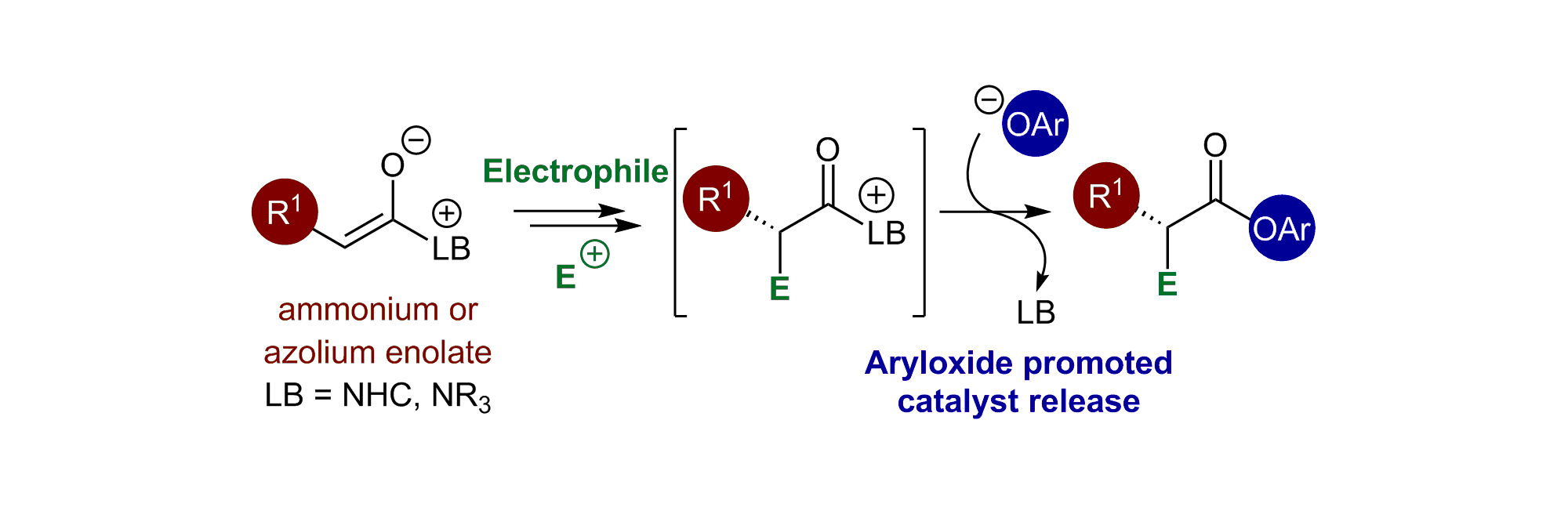
84. Aryloxide Promoted Catalyst Turnover in Lewis Base Organocatalysis
W. C. Hartley, T. J. C. O’Riordan and A. D. Smith
83. Catalytic Enantioselective [2,3]-Rearrangements of Allylic Ammonium Ylides: A Mechanistic and Computational Study
T. H. West, D. M. Walden, J. E. Taylor, A. C. Brueckner, R. C. Johnson, P. H.-Y. Cheong, G. C. Lloyd-Jones and A. D. Smith
82. Isothiourea-catalysed chemo- and enantioselective [2,3]-sigmatropic rearrangements of N,N-diallyl allylic ammonium ylides
T. H. West, S. S. M. Spoehrle and A. D. Smith
81. Enantioselective NHC-Catalysed Redox [4+2]-Hetero-Diels-Alder Reactions using α-Aroyloxyaldehydes and Unsaturated Ketoesters
J. E. Taylor, A. T. Davies, J. J. Douglas, G. Churchill and A. D. Smith
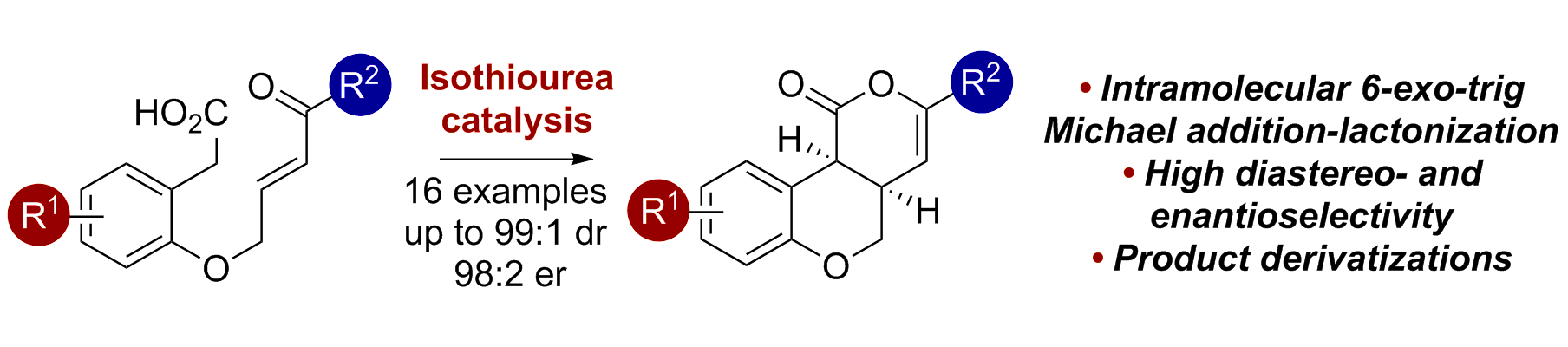
80. 6-exo-trig Michael Addition-Lactonizations for Catalytic Enantioselective Chromenone Synthesis
R. M.Neyyappadath, D. B.Cordes, A. M. Z. Slawin and A. D. Smith
79. Enantioselective N-heterocyclic carbene catalyzed formal [3+2] cycloaddition using α-aroyloxyaldehydes and oxaziridines
R. W. F. Kerr, M. D. Greenhalgh, A. M. Z. Slawin, P. L. Arnold and A. D. Smith
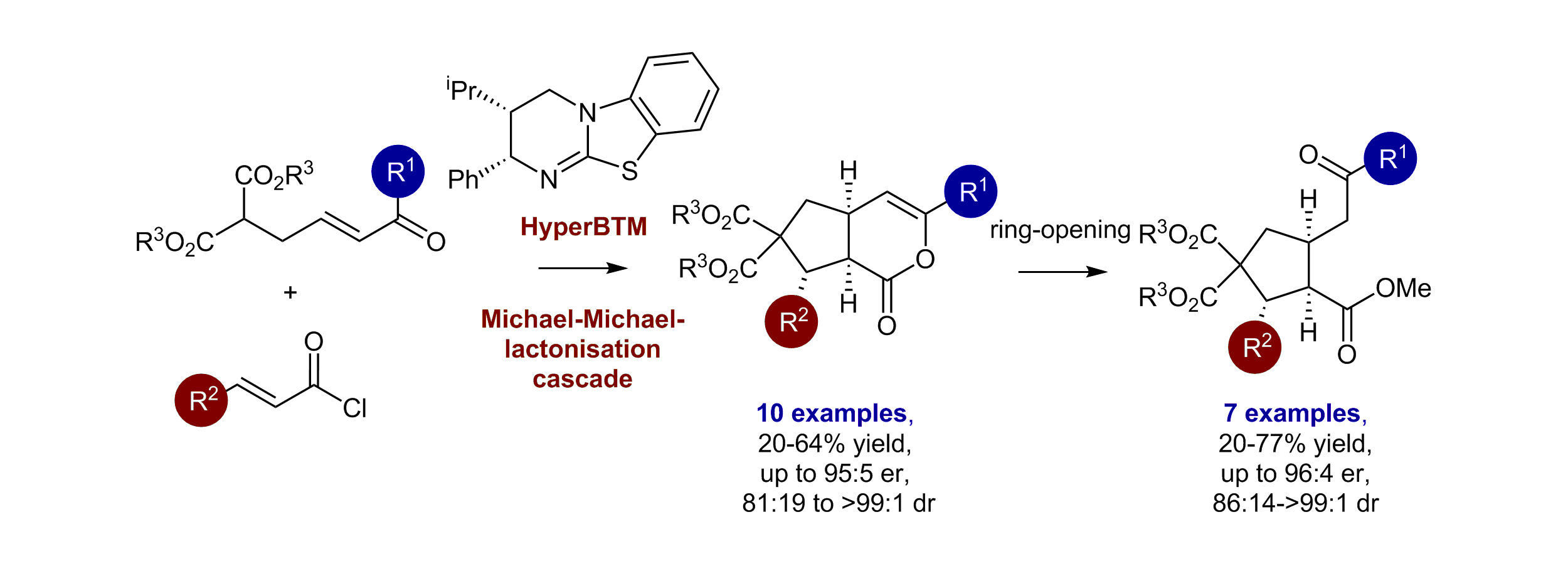
78. Enantioselective Isothiourea Catalysed Michael-Michael-Lactonisation Cascade; Synthesis of δ-Lactones and 1,2,3,4-Substituted Cyclopentanes
E. R. T. Robinson, A. B. Frost, P. Elías-Rodríguez and A. D. Smith
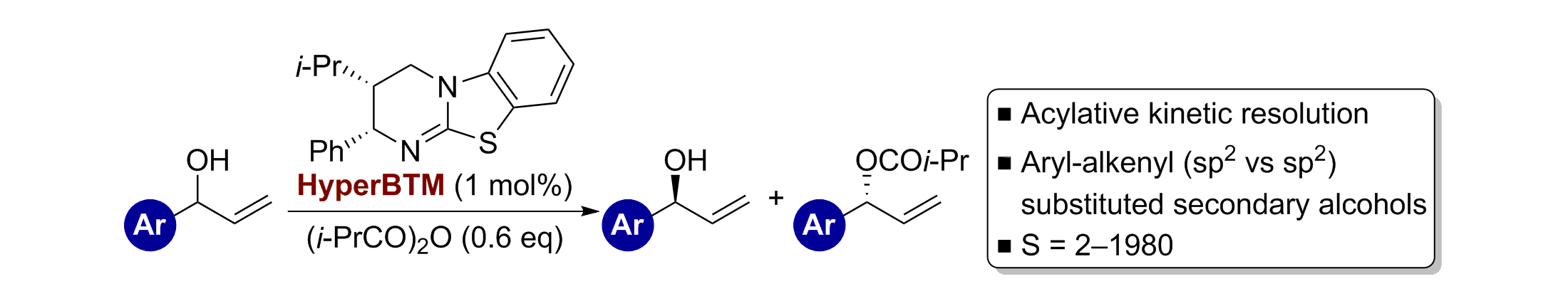
77. Isothiourea-Catalysed Acylative Kinetic Resolution of Aryl-Alkenyl (sp2 vs sp2) Substituted Secondary Alcohols
S. F. Musolino, O. S. Ojo, N. J. Westwood, J. E. Taylor and A. D. Smith
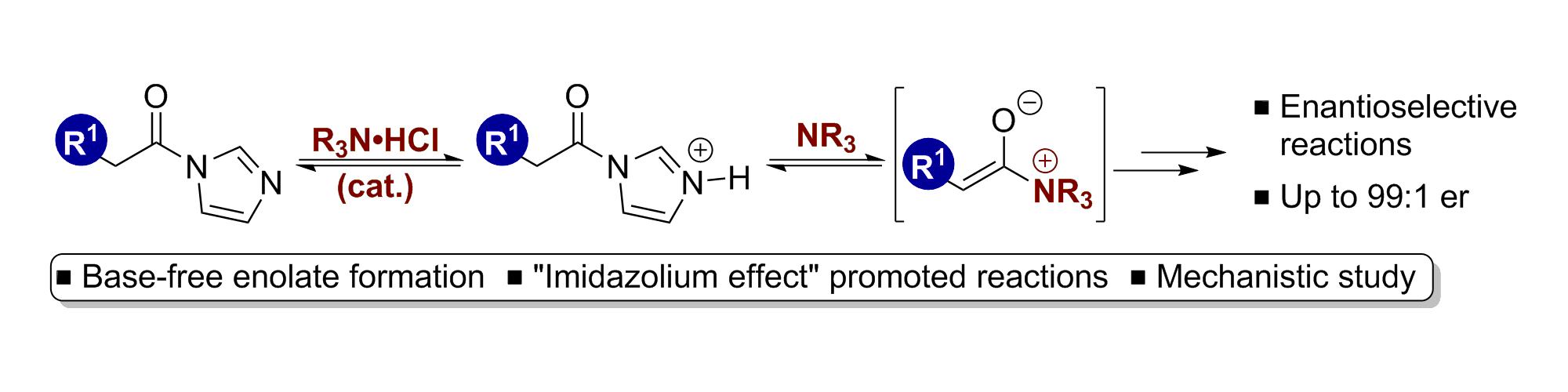
76. Exploiting the Imidazolium Effect in Base-free Ammonium Enolate Generation: Synthetic and Mechanistic Studies
C. M. Young, D. G. Stark, T. H. West, J. E. Taylor and A. D. Smith
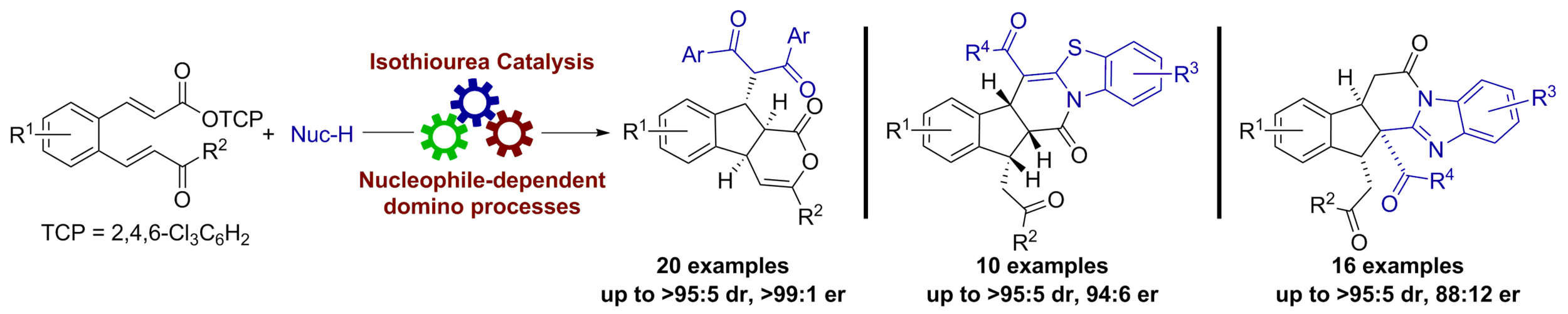
75. Enantioselective Stereodivergent Nucleophile-Dependent Isothiourea-Catalysed Domino Reactions
A. Matviitsuk, J. E. Taylor, D. B. Cordes, A. M. Z. Slawin and A. D. Smith
74. Enantioselective Isothiourea-Catalysed trans-Dihydropyridinone Synthesis using Saccharin-derived Ketimines: Scope and Limitations
D. G. Stark, C. M. Young, T. J. C. O'Riordan, A. M. Z. Slawin and A. D. Smith
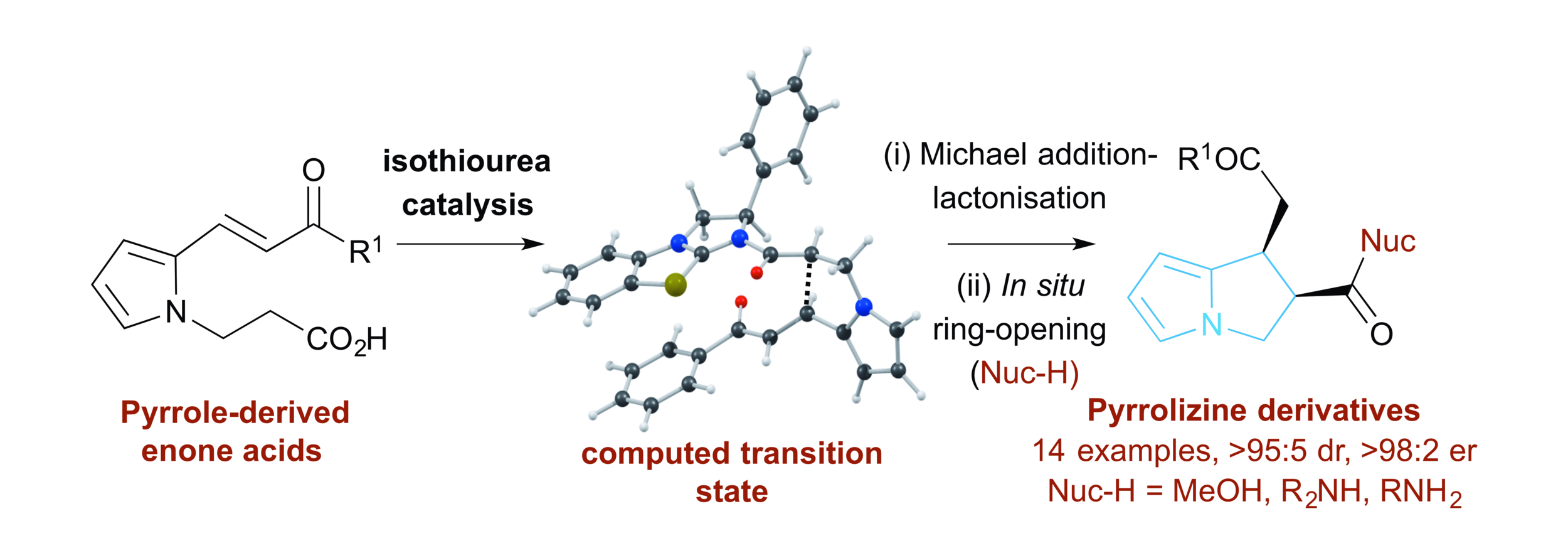
73. Catalytic Enantioselective Synthesis of Pyrrolizine Carboxylates using Isothiourea Catalysis: A Synthetic and Computational Study
D. G. Stark, P. Williamson, E. R. Gayner, S. F. Musolino, R. W. F. Kerr, J. E. Taylor, A. M. Z. Slawin, T. J. C. O'Riordan, S. A. MacGregor and A. D. Smith
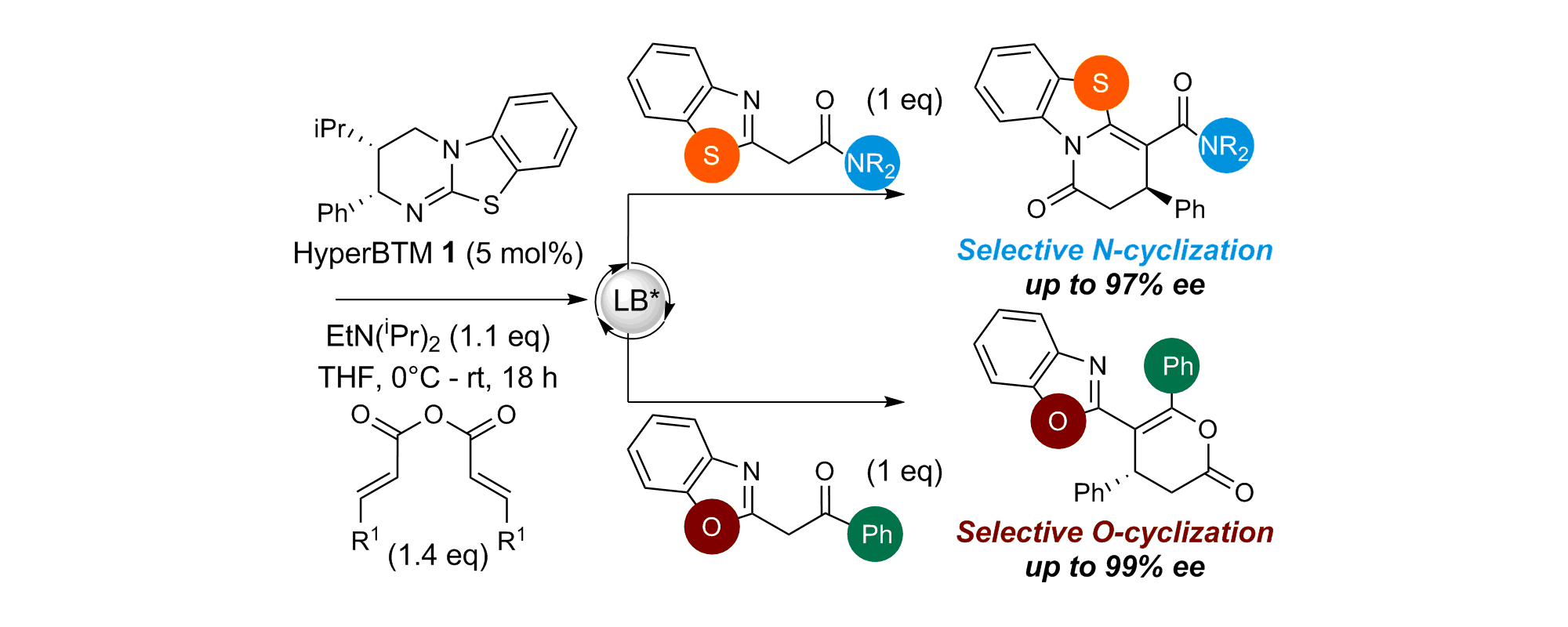
72. Non-Bonding 1,5-S•••O Interactions Govern Chemo- and Enantioselectivity in Isothiourea-Catalyzed Annulations of Benzazoles
E. R. T. Robinson, D. M. Walden, C. Fallan, M. D. Greenhalgh, P. H.-Y. Cheong and A. D. Smith
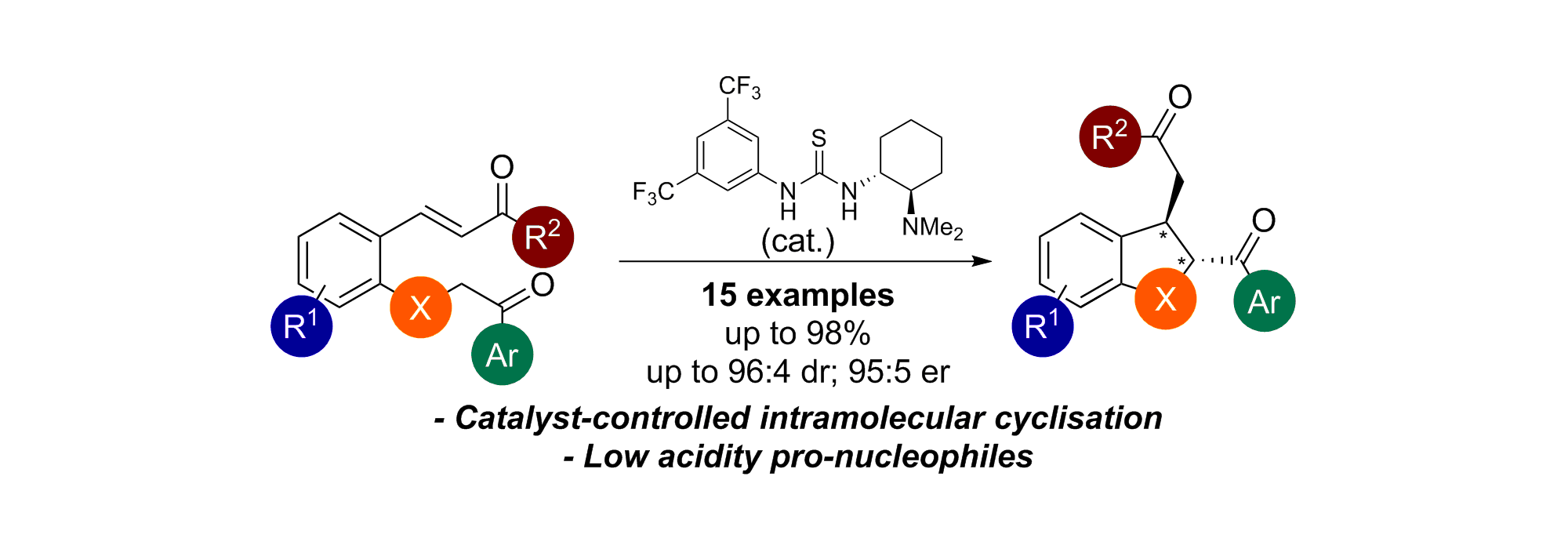
71. Enantioselective Synthesis of 2,3-Disubstituted trans-2,3-Dihydrobenzofurans Using a Brønsted Base/Thiourea Bifunctional Catalyst
D. -J. Barrios Antúnez, M. D. Greenhalgh, C. Fallan, A. M. Z. Slawin and A. D. Smith
70. Quinidine-Catalysed Enantioselective Synthesis of 6- and 4-Trifluoromethyl-Substituted Dihydropyrans
K. Kasten, D. B. Cordes, A. M. Z. Slawin and A. D. Smith
69. Isothiourea-Mediated Organocatalytic Michael Addition–Lactonization on a Surface: Modification of SAMs on Silicon Oxide Substrates
R. Chisholm, J. D. Parkin, A. D. Smith and G. Hähner
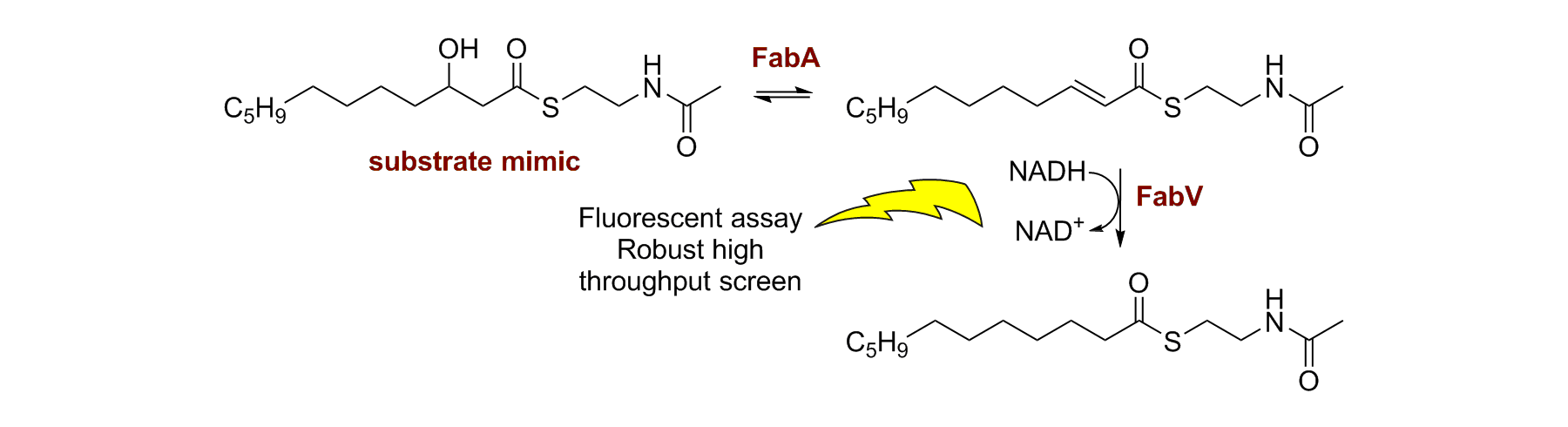
68. A Substrate Mimic Allows High-Throughput Assay of the FabA Protein and Consequently the Identification of a Novel Inhibitor of Pseudomonas aeruginosa FabA
L. Moynie, A. G. Hope, K. Finzel, J. Schmidberger, S. M. Leckie, G. Schneider, M. D. Burkhart, A. D. Smith, D. W. Gray and J. H. Naismith
67. Enantioselective Synthesis of 3,5,6-Substituted Dihydropyranones and Dihydropyridinones using Isothiourea-Mediated Catalysis
D. G. Stark, L. C. Morrill, D. B. Cordes, A. M. Z. Slawin, T. J. C. O'Riordan and A. D. Smith
66. Organocatalytic Synthesis of Fused Bicyclic 2,3-Dihydro-1,3,4-oxadiazoles through an Intramolecular Cascade Cyclization
A. J. Fugard, B. K. Thompson, A. M. Z. Slawin, J. E. Taylor and A. D. Smith
65. Catalytic Stereoselective [2,3]-Rearrangement Reactions
T. H. West, S. S. M. Spoehrle, K. Kasten, J. E. Taylor and A. D. Smith
64. Enantioselective NHC-Catalyzed Redox [2+2] Cycloadditions with Perfluoroketones; A Route to Fluorinated Oxetanes
A. T. Davies, A. M. Z. Slawin and A. D. Smith
63. Stereo- and Chemodivergent NHC-Promoted Functionalisation of Arylalkylketenes with Chloral
J. J. Douglas, G. Churchill, A. M. Z. Slawin, D. J. Fox and A. D. Smith
62. Enantioselective NHC-Catalyzed Redox [4+2]-hetero-Diels-Alder Reactions using alpha,beta-Unsaturated Trichloromethyl ketones as Amide Equivalents
N. Attaba, J. E. Taylor, A. M. Z. Slawin and A. D. Smith
61. Asymmetric Isothiourea-Catalysed Formal [3+2] Cycloadditions of Ammonium Enolates with Oxaziridines
S. R. Smith, C. Fallan, J. E. Taylor, R. McLennan, D. S. B. Daniels, L. C. Morrill, A. M. Z. Slawin and A. D. Smith
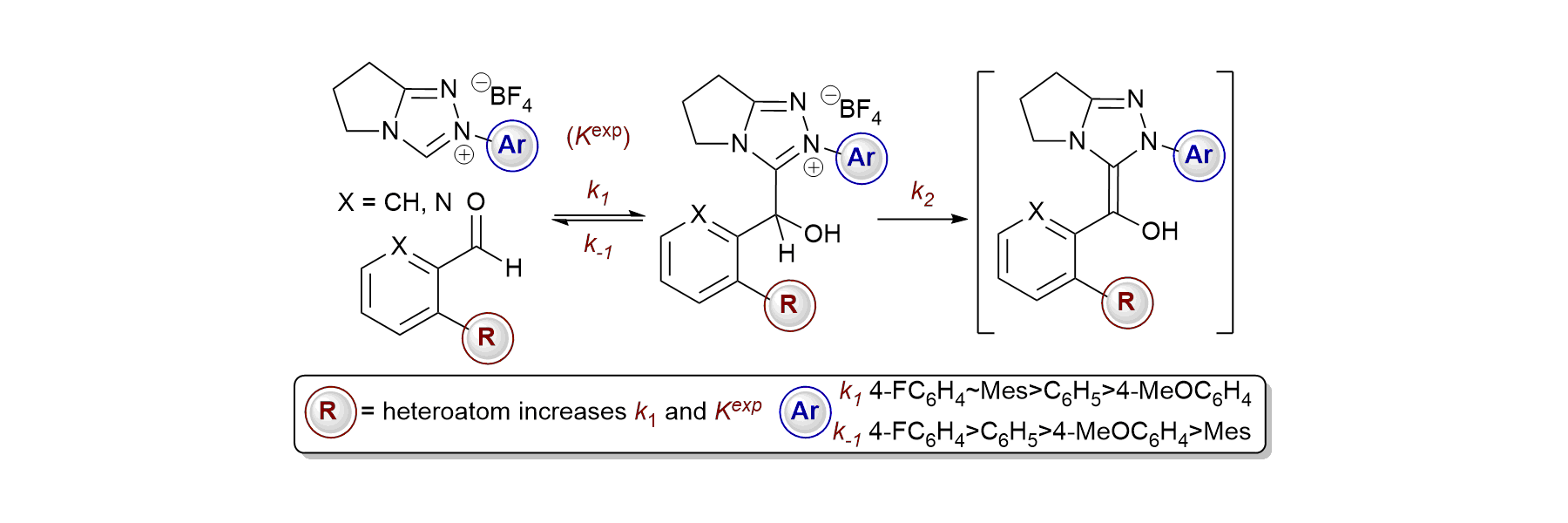
60. Rate and Equilibrium Constants for the Addition of N-Heterocyclic Carbenes into Benzaldehydes: A Remarkable 2-Substituent Effect
C. J. Collett, R. S. Massey, J. E. Taylor, O. R. Maguire, A. C. O'Donoghue and A. D. Smith
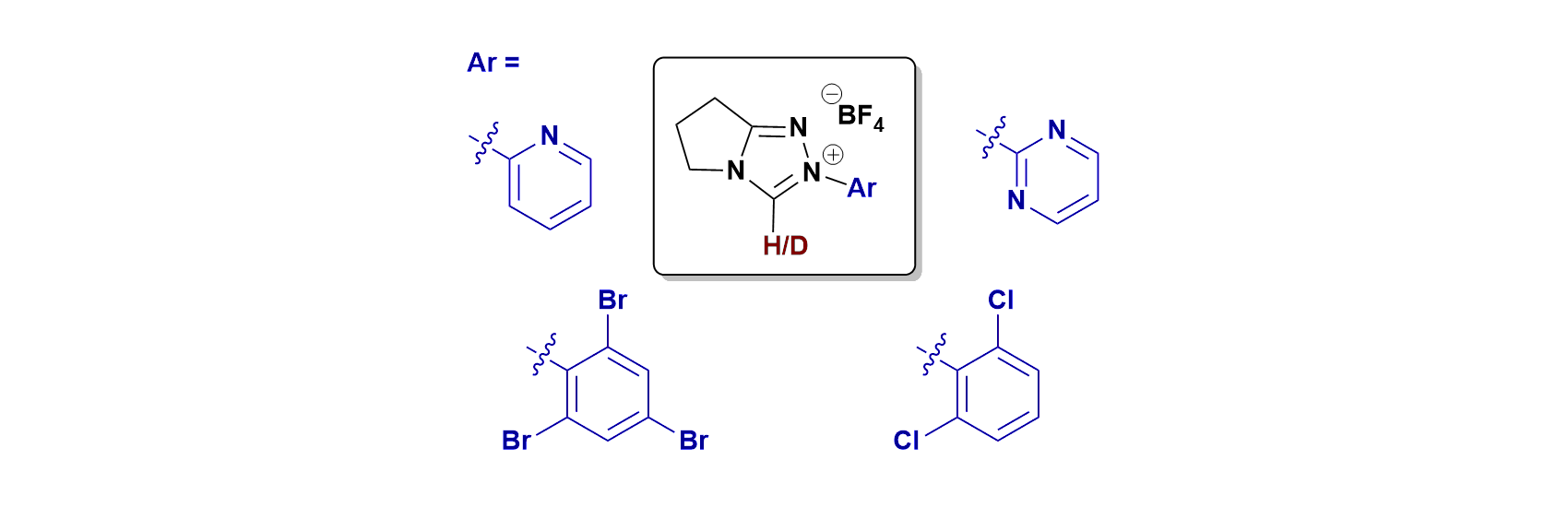
59. Proton transfer reactions of N-aryl triazolium salts: unusual ortho-substituent effects
D. E. Tucker, P. Quinn, R. S. Massey, C. J. Collett, D. J. Jasiewicz, C. R. Bramley, A. D. Smith and A. C. O'Donoghue
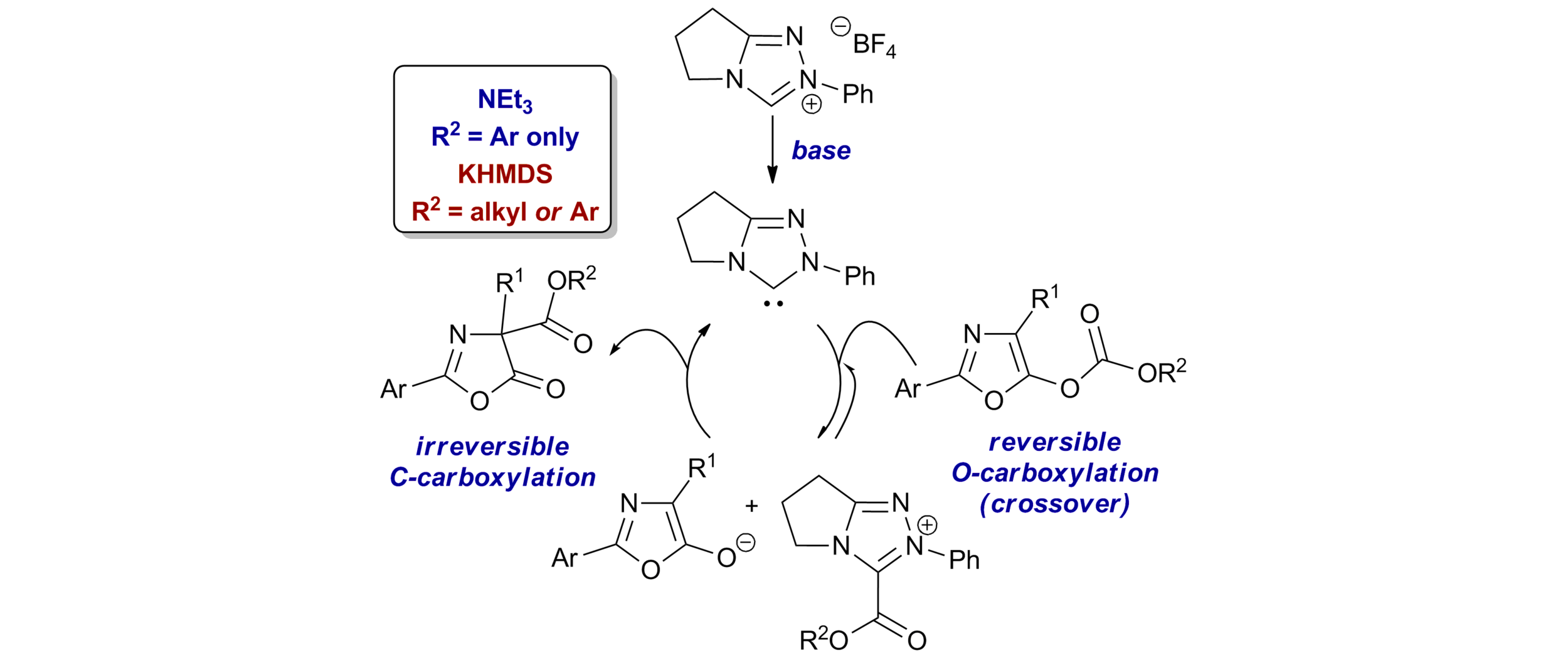
58. Regiodivergent Lewis base-promoted O- to C-carboxyl transfer of furanyl carbonates
C. D. Campbell, C. Joannesse, L. C. Morrill, D. Philp and A. D. Smith
57. Exploring the scope of the isothiourea-mediated synthesis of dihydropyridinones
P.-P. Yeh, D. S. B. Daniels, C. Fallan, E. Gould, C. Simal, J. E. Taylor, A. M. Z. Slawin and A. D. Smith
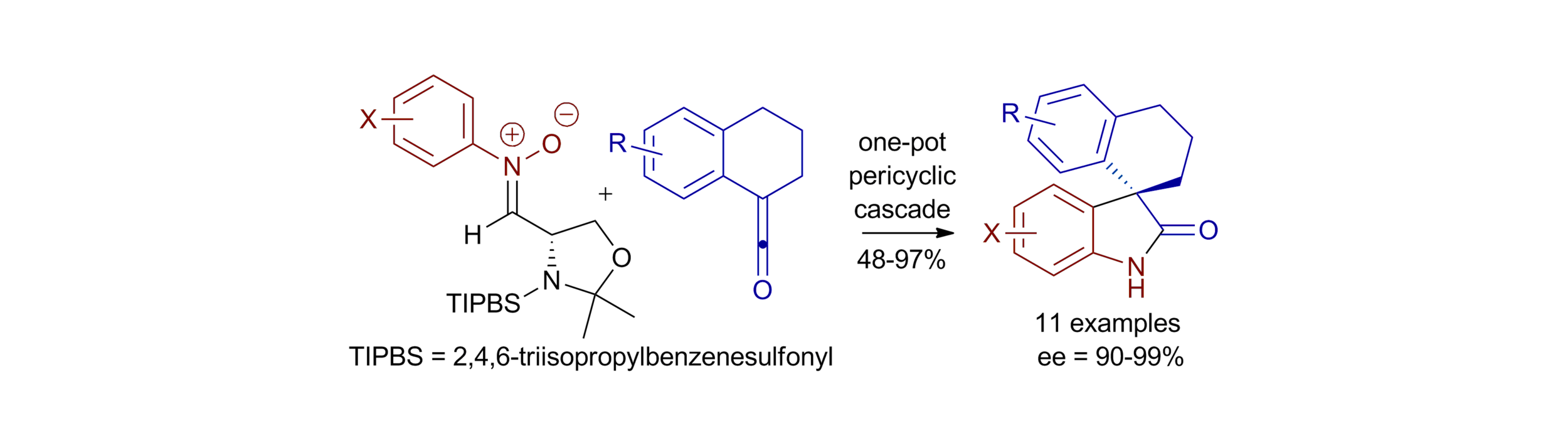
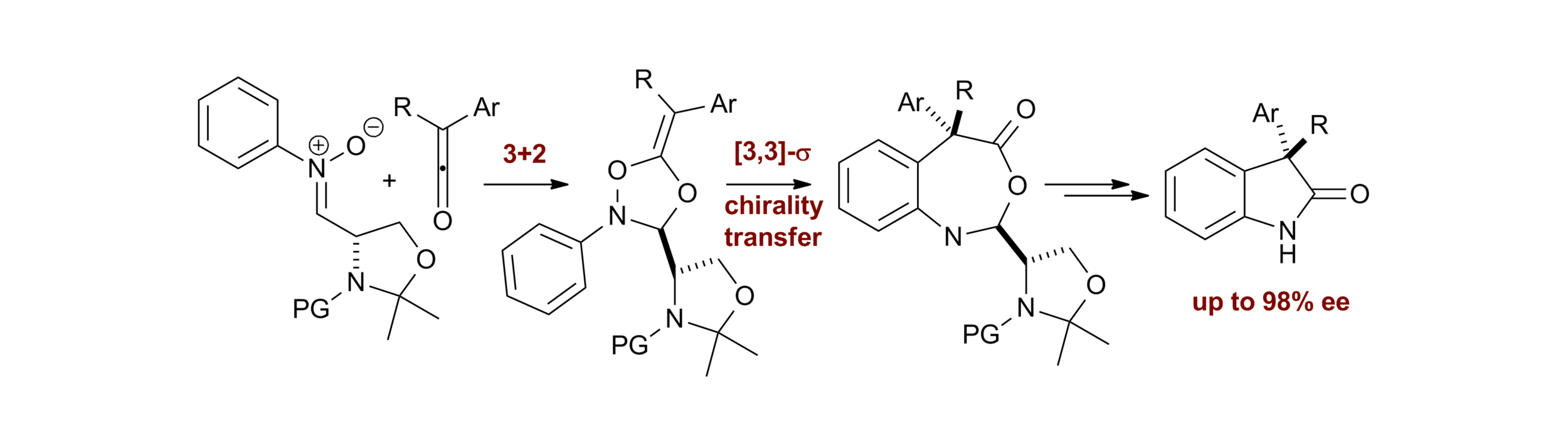
56. An asymmetric pericyclic cascade approach to 3-alkyl-3-aryloxindoles: generality, applications and mechanistic investigations
E. Richmond, K. B. Ling, N. Duguet, L. B. Manton, N. Çelebi-Ölçüm, Y.-H. Lam, S. Alsancak, A. M. Z. Slawin, K. N. Houk and A. D. Smith
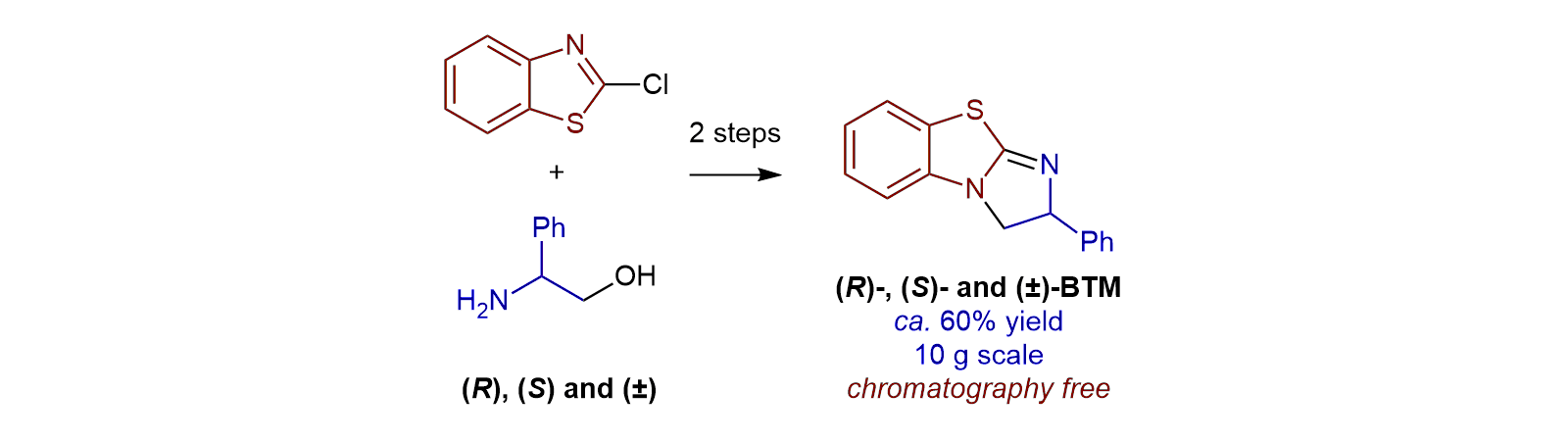
55. A Scalable, Chromatography-Free Synthesis of Benzotetramisole
D. S. B. Daniels, S. R. Smith, T. Lebl, P. Shapland and A. D. Smith
54. Synthesis of Di-, Tri-, and Tetrasubstituted Pyridines from (Phenylthio)carboxylic Acids and 2-[Aryl(tosylimino)methyl]acrylates
D. G. Stark, T. J. C. O'Riordan and A. D. Smith
53. Organocatalytic Michael addition–lactonisation of carboxylic acids using α,β-unsaturated trichloromethyl ketones as α,β-unsaturated ester equivalents
L. C. Morrill, D. G. Stark, J. E. Taylor, S. R. Smith, J. A. Squires, A. C. A. D'Hollander, C. Simal, P. Shapland, T. J. C. O'Riordan and A. D. Smith
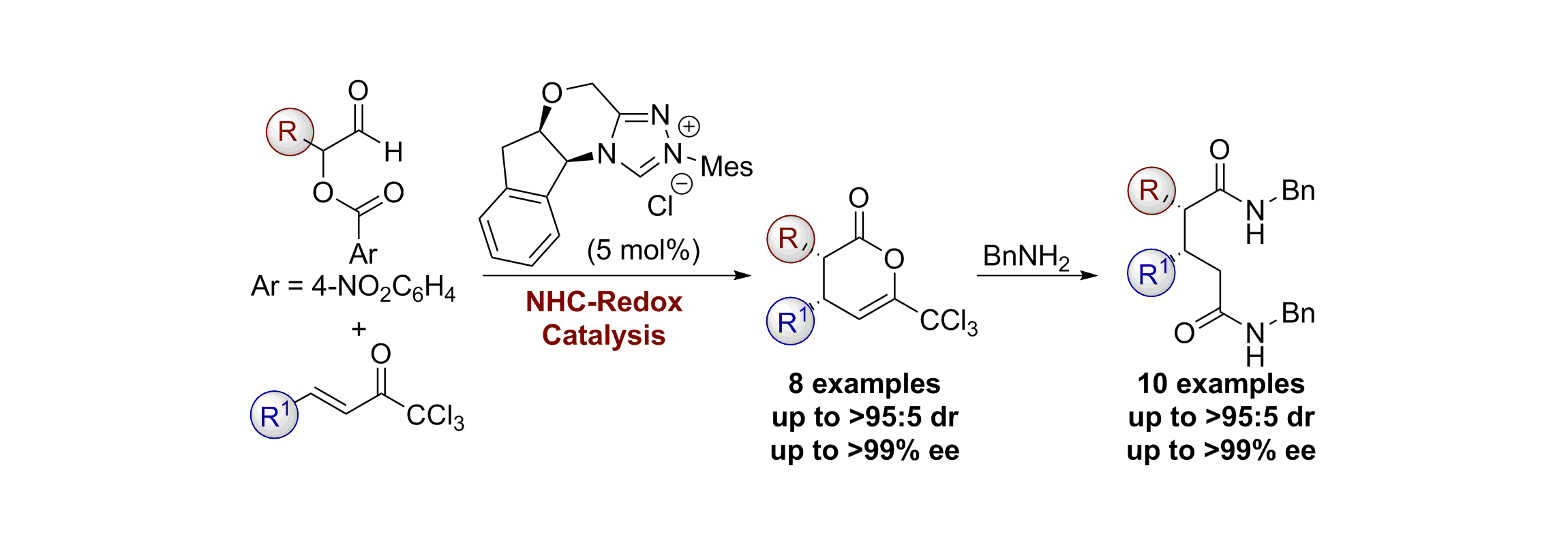
52. Asymmetric Synthesis of Tri- and Tetrasubstituted Trifluoromethyl Dihydropyranones from α-Aroyloxyaldehydes via NHC-Redox Catalysis
A. T. Davies, P. M. Pickett, A. M. Z. Slawin and A. D. Smith
51. Stereodivergent Organocatalytic Intramolecular Michael Addition/Lactonization for the Asymmetric Synthesis of Substituted Dihydrobenzofurans and Tetrahydrofurans
D. Belmessieri, A. de la Houpliere, E. D. D. Calder, J. E. Taylor and A. D. Smith
50. Catalyst selective and regiodivergent O- to C- or N-carboxyl transfer of pyrazolyl carbonates: synthetic and computational studies
E. Gould, D. M. Walden, K. Kasten, R. C. Johnston, J. Wu, A. M. Z. Slawin, T. J. L. Mustard, B. Johnston, T. Davies, P. H.-Y. Cheong and A. D. Smith
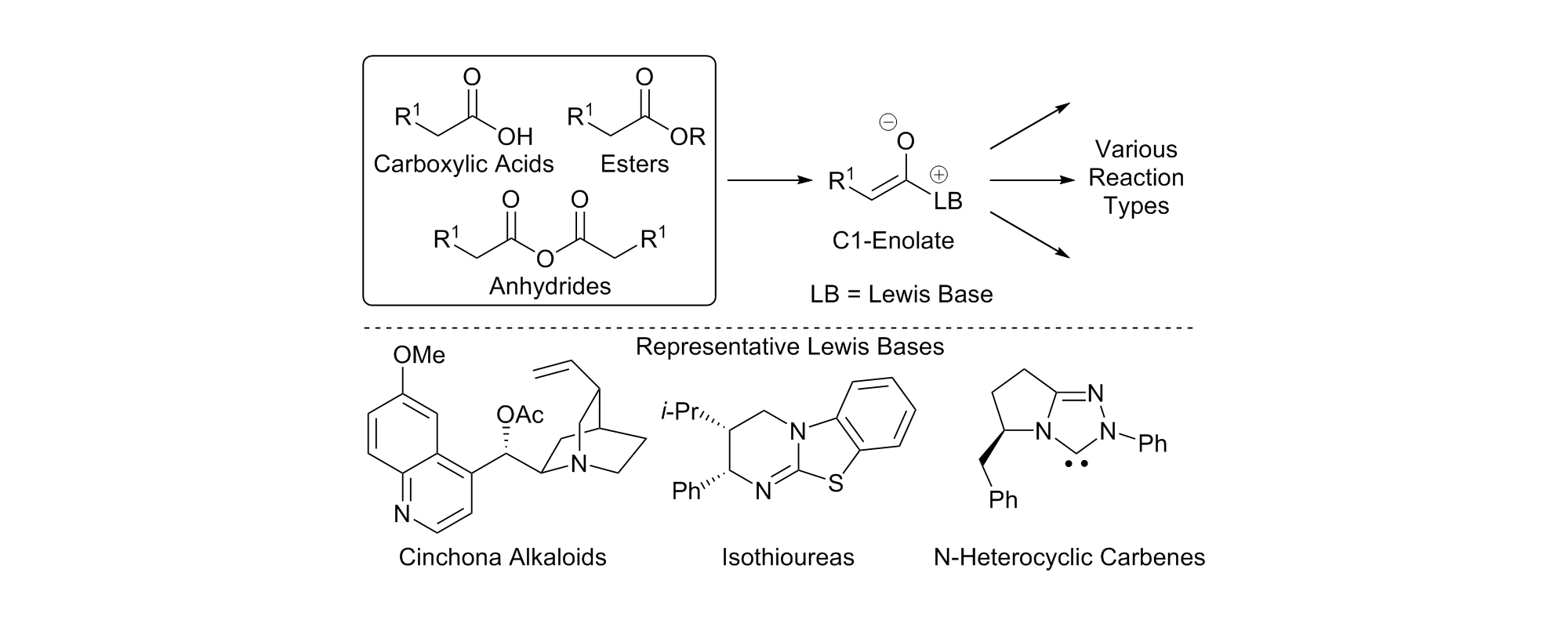
49. Organocatalytic Lewis base functionalisation of carboxylic acids, esters and anhydrides via C1-ammonium or azolium enolates
L. C. Morrill and A. D. Smith
48. α-Ketophosphonates as Ester Surrogates: Isothiourea-Catalyzed Asymmetric Diester and Lactone Synthesis
S. R. Smith, S. M. Leckie, R. Holmes, J. Douglas, C. Fallan, P. Shapland, D. Pryde, A. M. Z. Slawin and A. D. Smith
47. An Isothiourea-catalyzed Asymmetric [2,3]-Rearrangement of Allylic Ammonium Ylides
T. H. West, D. S. B. Daniels, A. M. Z. Slawin and A. D. Smith
46. Isothiourea-Mediated Asymmetric Functionalization of 3-Alkenoic Acids
L. C. Morrill, S. M. Smith, A. M. Z. Slawin and A. D. Smith
45. Isothiourea-catalyzed asymmetric synthesis of beta-lactams and beta-amino esters from arylacetic acid derivatives and N-sulfonyl aldimines
S. R. Smith, J. Douglas, H. Prevet, P. Shapland, A. M. Z. Slawin and A. D. Smith
44. Isothiourea-Mediated One-Pot Synthesis of Trifluoromethyl Substituted 2-Pyrones
P.-P. Yeh, D. S. B. Daniels, D. B. Cordes, A. M. Z. Slawin and A. D. Smith
43. 2-Arylacetic anhydrides as ammonium enolate precursors
L. C. Morrill, L. A. Ledingham, J.-P. Couturier, J. Bickel, A. D. Harper, C. Fallan and A. D. Smith
42. Asymmetric NHC-Catalyzed Redox α-Amination of α-Aroyloxyaldehydes
J. E. Taylor, D. S. B. Daniels and A. D. Smith
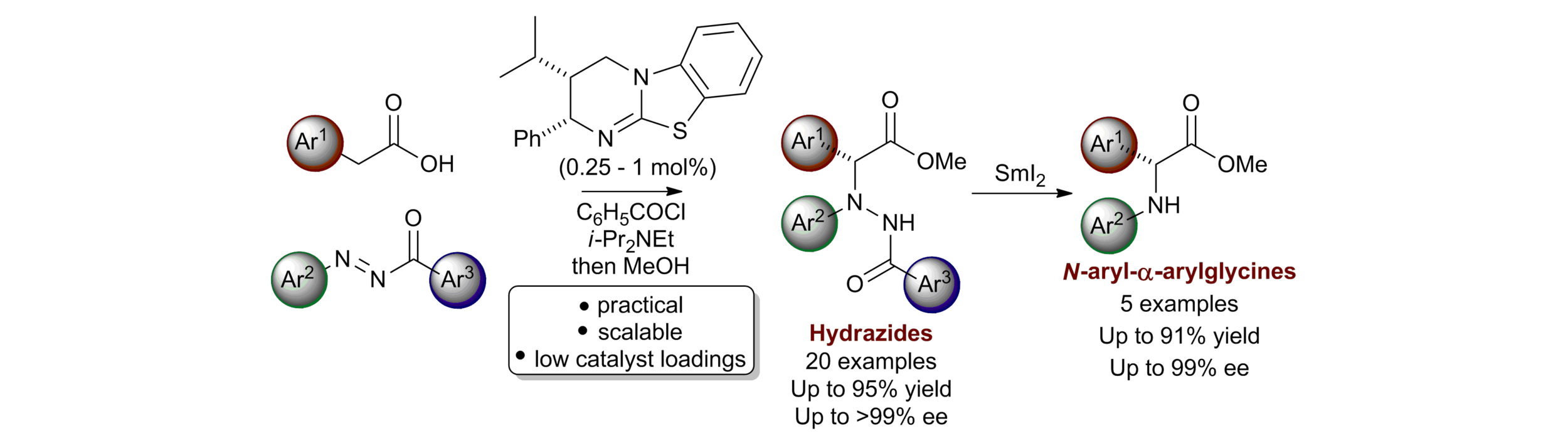
41. The development of highly active acyclic chiral hydrazides for asymmetric iminium ion organocatalysis
E. Gould, T. Lebl, A. M. Z. Slawin, M. Reid, T. Davies and A. D. Smith
40. Isothiourea-Mediated One-Pot Synthesis of Functionalized Pyridines
D. G. Stark, L. C. Morrill, P.-P. Yeh, A. M. Z. Slawin, T. J. C. O'Riordan and A. D. Smith
39. Stereospecific Asymmetric N-Heterocyclic Carbene (NHC)-Catalyzed Redox Synthesis of Trifluoromethyl Dihydropyranones and Mechanistic Insights
A. T. Davies, J. E. Taylor, J. Douglas, C. J. Collett, L. C. Morrill, C. Fallan, A. M. Z. Slawin, G. Churchill and A. D. Smith
38. Isothiourea-mediated asymmetric Michael-lactonisation of trifluoromethylenones: a synthetic and mechanistic study
L. C. Morrill, J. Douglas, T. Lebl, A. M. Z. Slawin, D. J. Fox and A. D. Smith
37. Telescoped Synthesis of Stereodefined Pyrrolidines
D. Belmessieri, D. B. Cordes, A. M. Z. Slawin and A. D. Smith
36. Enantioselective NHC-Catalysed Formal [4+2] Cycloaddition of Alkylarylketenes with β,γ-Unsaturated α-Ketophosphonates
S. M. Leckie, C. Fallan, J. E. Taylor, T. B. Brown, D. Pryde, T. Lebl, A. M. Z. Slawin and A. D. Smith
35. NHC-mediated enantioselective formal [4+2] cycloadditions of alkylarylketenes and β,γ-unsaturated α-ketocarboxylic esters and amides
S. M. Leckie, T. B. Brown, D. Pryde, T. Lebl, A. M. Z. Slawin and A. D. Smith
34. NHC-Promoted Asymmetric β-Lactone Formation from Arylalkylketenes and Electron-Deficient Benzaldehydes or Pyridinecarboxaldehydes
J. Douglas, J. E. Taylor, G. Churchill, A. M. Z. Slawin and A. D. Smith
33. Anhydrides as α,β-unsaturated acyl ammonium precursors: Isothiourea-promoted catalytic asymmetric annulation processes
E. R. T. Robinson, C. Fallan, C. Simal, A. M. Z. Slawin and A. D. Smith
32. Mechanistic insights into the triazolylidene-catalysed Stetter and Benzoin Reactions: role of the N-aryl substituent
C. J. Collett, R. S. Massey, O. R. Maguire, A. S. Batsanov, A. C. O'Donoghue and A. D. Smith
31. Structural insights into the mechanism and inhibition of the β-hydroxydecanoyl-acyl carrier protein dehydratase from Pseudomonas aeruginosa
L. Moynié, S. M. Leckie, S. A. McMahon, F. G. Duthie, A. Koehnke, J. W. Taylor, M. S. Alphey, R. Brenk, A. D. Smith and J. H. Naismith
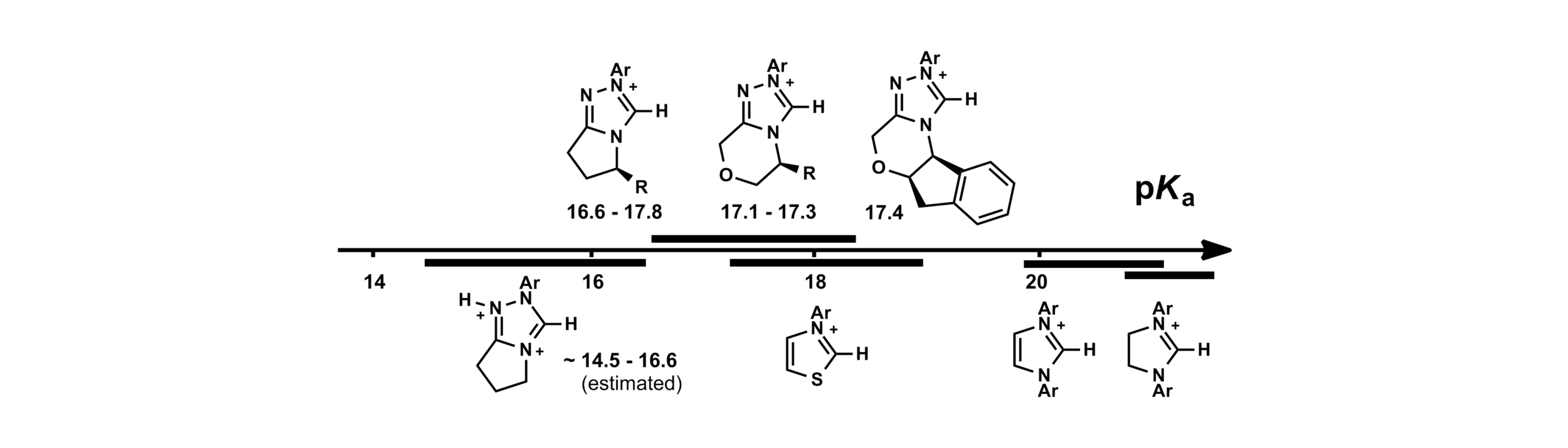
30. Proton Transfer Reactions of Triazol-3-ylidenes: Kinetic Acidities and Carbon Acid pKa Values for Twenty Triazolium Salts in Aqueous Solution
R. S. Massey, C. J. Collett, A. G. Lindsay, A. D. Smith and A. C. O'Donoghue
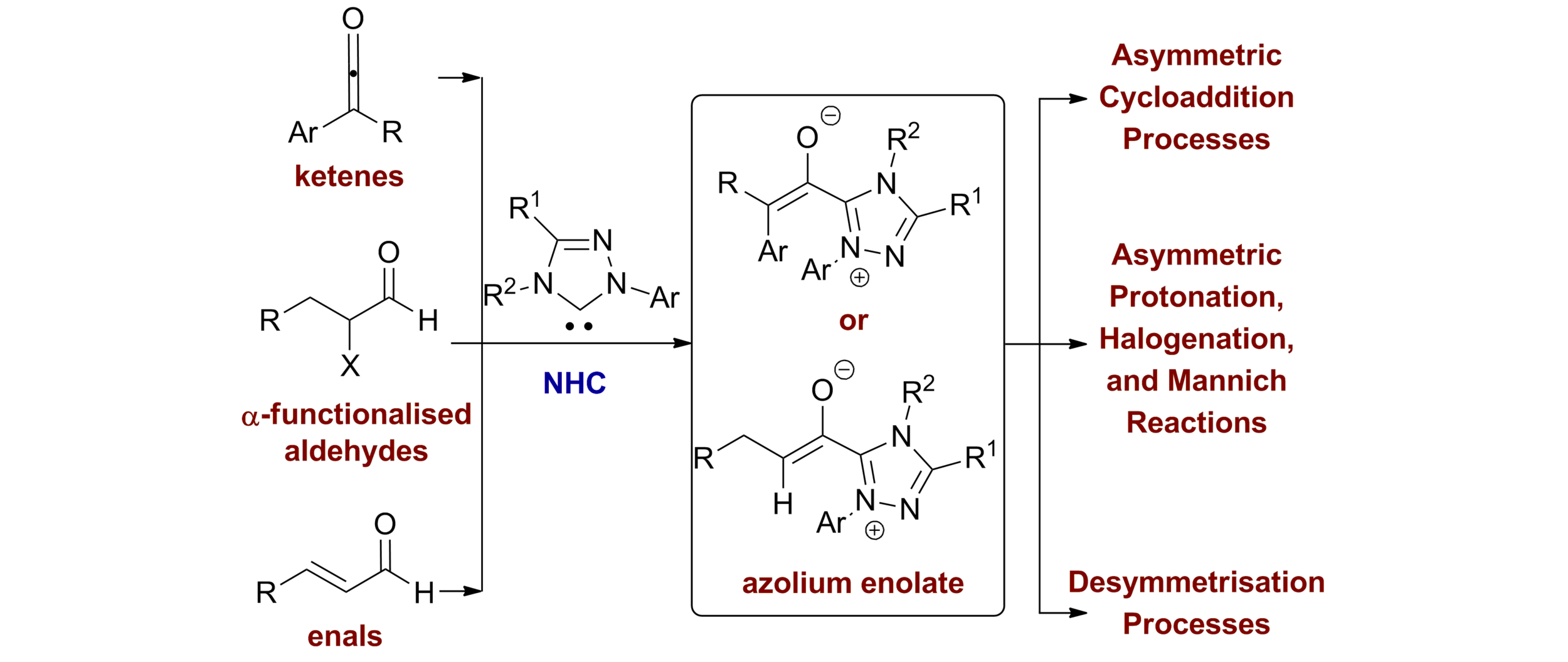
29. NHCs in Asymmetric Organocatalysis: Recent Advances in Azolium Enolate Generation and Reactivity
J. Douglas, G. Churchill and A. D. Smith
28. Asymmetric Pericyclic Cascade Approach to Spirocyclic Oxindoles
E. Richmond, N. Duget, A. M. Z. Slawin, T. Lebl and A. D. Smith
27. Catalytic asymmetric α-amination of carboxylic acids using isothioureas
L. Morrill, T. Lebl, A. M. Z. Slawin and A. D. Smith
26. Dihydropyridones: catalytic asymmetric synthesis, N- to C-sulfonyl transfer and derivatizations
C. Simal, T. Lebl, A. M. Z. Slawin and A. D. Smith
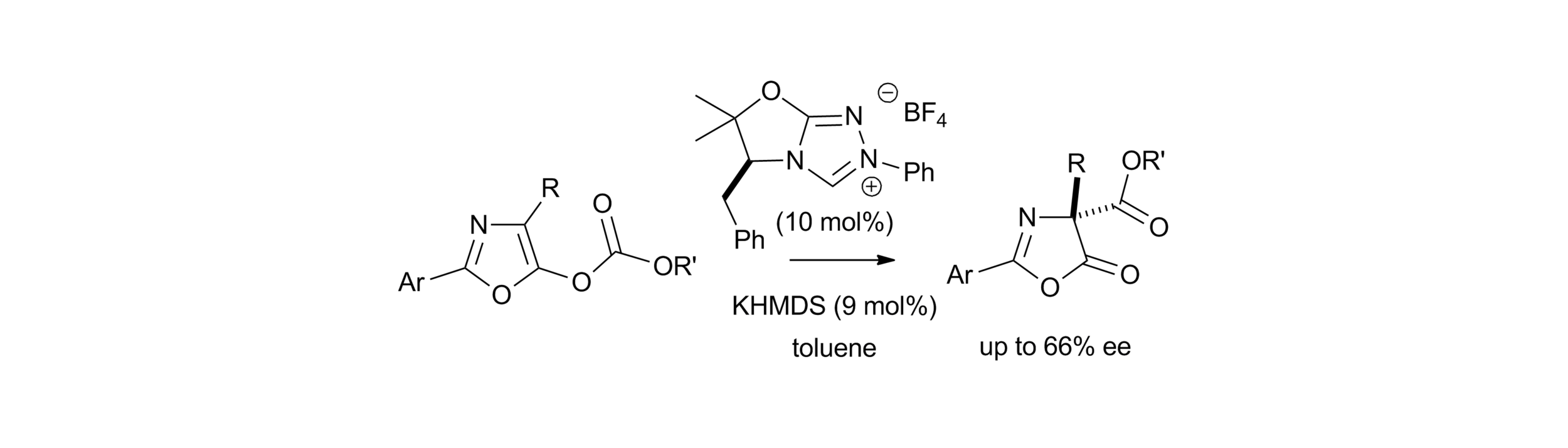
25. Isothiourea-mediated asymmetric O- to C-carboxyl transfer of oxazolyl carbonates: structure-selectivity profiles and mechanistic studies
C. Joannesse, C. P. Johnston, L. C. Morrill, P. A. Woods, M. Kieffer, T. A. Nigst, H. Mayr, T. Lebl, D. Philp, R. A. Bragg and A. D. Smith
24. Pericyclic Cascade with Chirality Transfer: Reaction Pathway and Origin of Enantioselectivity of the Hetero-Claisen Approach to Oxindoles
N. Çelebi-Ölçüm, Y. Lam, E. Richmond, K. B. Ling, A. D. Smith and K. N. Houk
23. Isothiourea-Catalyzed Asymmetric C-acylation of Silyl Ketene Acetals
P. A. Woods, L. C. Morrill, R. A. Bragg and A. D. Smith
22. Nucleophilicities and Lewis Basicities of Isothiourea Derivatives
B. Maji, C. Joannesse, T. Nigst, A. D. Smith and H. Mayr
21. Isothiourea-catalyzed asymmetric O- to C-carboxyl transfer of furanyl carbonates
C. Joannesse, L. C. Morrill, C. D. Campbell, A. M. Z. Slawin and A. D. Smith
20. Catalytic Enantioselective Steglich Rearrangements using Chiral N-Heterocyclic Carbenes
C. D. Campbell, C. Concellón and A. D. Smith
19. Organic base effects in NHC promoted O- to C-carboxyl transfer; chemoselectivity profiles, mechanistic studies and domino catalysis
C. D. Campbell, C. J. Collett, J. E. Thomson, A. M. Z. Slawin and A. D. Smith
18. Organocatalytic Functionalization of Carboxylic acids: Isothiourea-Catalyzed Asymmetric Intra- and Intermolecular Michael Addition-Lactonizations
D. Belmessieri, L. C. Morrill, C. Simal, A. M. Z. Slawin and A. D. Smith
17. Structure-enantioselectivity effects in 3,4-dihydropyrimido[2,1-b]benzothiazole-based isothioureas as enantioselective acylation catalysts
D. Belmessieri, C. Joannesse, P. A. Woods, C. MacGregor, C. Jones, C. D. Campbell, C. P. Johnston, N. Duguet, C. Concellón, R. A. Bragg and A. D. Smith
16. α-Aroyloxyaldehydes; scope and limitations as alternatives to α-haloaldehydes for NHC-catalysed redox transformations
K. B. Ling and A. D. Smith
15. Structural effects in pyrazolidinone-mediated iminium ion-promoted Diels-Alder reactions
E. Gould, M. Reid, A. M. Z. Slawin and A. D. Smith
14. NHC-mediated chlorination of alkylarylketenes: Catalysis and Asymmetry
J. Douglas, K. B. Ling, C. Concellón, G. Churchill, A. M. Z. Slawin and A. D. Smith
13. Isothiourea-Mediated Stereoselective C-Acylation of Silyl Ketene Acetals
P. A. Woods, L. C. Morrill, T. Lebl, A. M. Z. Slawin, R. A. Bragg, and A. D. Smith
12. Chiral relay in NHC-mediated asymmetric β-lactam synthesis II; substituent effects in NHCs derived from acylic 1,2-diamines
N. Duguet, A. Donaldson, S. M. Leckie, E. A. Kallstrom, C. D. Campbell, P. Shapland, T. B. Brown, A. M. Z. Slawin and A. D. Smith
11. Chiral relay in NHC-mediated asymmetric β-lactam synthesis I; substituent effects in NHCs derived from (1R,2R)-cyclohexyl-1,2-diamine
N. Duguet, A. Donaldson, S. M. Leckie, J. Douglas, P. Shapland, T. B. Brown, G. Churchill, A. M. Z. Slawin and A. D. Smith
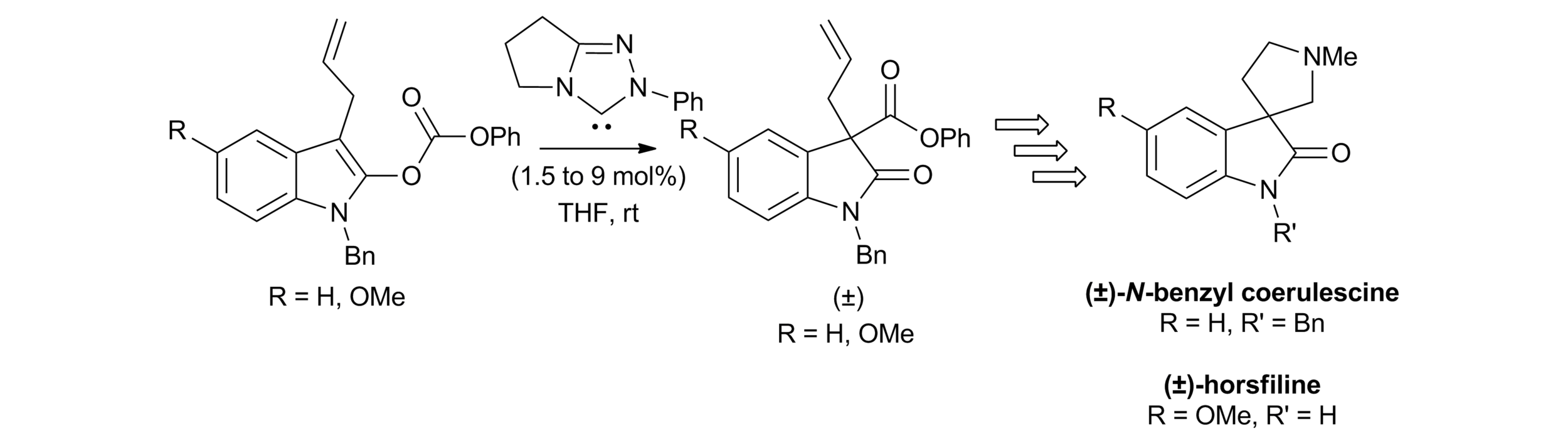
10. Applications of NHC-mediated O- to C-carboxyl transfer: synthesis of (±)-N-benzyl-coerulescine and (±)-horsfiline
J. E. Thomson, A. F. Kyle, K. B. Ling, S. R. Smith, A. M. Z. Slawin and A. D. Smith
9. NHC-mediated enantioselective addition of phenols to unsymmetrical alkylarylketenes
C. Concellón, N. Duguet and A. D. Smith
8. Isothiourea catalysed enantioselective O- to C-carboxyl transfer
C. Joannesse, C. P. Johnston, C. Concellón, C. Simal, D. Philp and A. D. Smith
7. An asymmetric hetero-Claisen approach to 3-alkyl-3-aryloxindoles
N. Duguet, A. M. Z. Slawin and A. D. Smith
6. N-heterocyclic carbene catalysed O- to C-carboxyl transfer of indolyl and benzofuranyl carbonates
J. E. Thomson, A. F. Kyle, C. Concellón, K. A. Gallagher, P. Lenden, L. C. Morrill, A. J. Miller, C. Joannesse, A. M. Z. Slawin and A. D. Smith
5. Amidine Catalysed O- to C-carboxyl transfer of heterocyclic carbonate derivatives
C. Joannesse, C. Simal, C. Concellón, J. E. Thomson, C. D. Campbell, A. M. Z. Slawin and A. D. Smith
4. Tandem multi-step synthesis of C-carboxyazlactones using N-heterocyclic carbenes
C. D. Campbell, N. Duguet, K. A. Gallagher, J. E. Thomson, A. G. Lindsay, A. O’Donoghue and A. D. Smith
3. Probing the efficiency of N-heterocyclic carbene promoted O- to C-carboxyl transfer of oxazolyl carbonates
J. E. Thomson, C. D. Campbell, C. Concellón, N. Duguet, K. Rix, A. M. Z. Slawin and A. D. Smith
2. N-Heterocyclic carbene catalysed β-lactam synthesis
N. Duguet, C. D. Campbell, A. M. Z. Slawin and A. D. Smith
1. Efficient N-Heterocyclic Carbene Catalysed O- to C-acyl transfer
J. E. Thomson, K. Rix and A. D. Smith

![128[15300].png](https://images.squarespace-cdn.com/content/v1/5cda8b5401232ca7bb453461/1618236416439-Y9I57GYM0BGL6FJ2DGS1/128%5B15300%5D.png)

![125[15299].png](https://images.squarespace-cdn.com/content/v1/5cda8b5401232ca7bb453461/1618398688309-HWD4LQJDBVJJDAO8M28Q/125%5B15299%5D.png)
![117[15431].png](https://images.squarespace-cdn.com/content/v1/5cda8b5401232ca7bb453461/1618405047762-9MHHTT695YCXEQR8I5X3/117%5B15431%5D.png)